Light Environment Influences Developmental Programming of the Metabolic and Visual Systems in Mice
- PMID: 33861321
- PMCID: PMC8083116
- DOI: 10.1167/iovs.62.4.22
Light Environment Influences Developmental Programming of the Metabolic and Visual Systems in Mice
Abstract
Purpose: Light is a salient cue that can influence neurodevelopment and the immune system. Light exposure out of sync with the endogenous clock causes circadian disruption and chronic disease. Environmental light exposure may contribute to developmental programming of metabolic and neurological systems but has been largely overlooked in Developmental Origins of Health and Disease (DOHaD) research. Here, we investigated whether developmental light exposure altered programming of visual and metabolic systems.
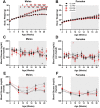
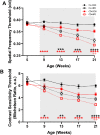
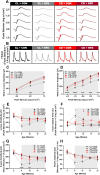
Methods: Pregnant mice and pups were exposed to control light (12:12 light:dark) or weekly light cycle inversions (circadian disruption [CD]) until weaning, after which male and female offspring were housed in control light and longitudinally measured to evaluate differences in growth (weight), glucose tolerance, visual function (optomotor response), and retinal function (electroretinogram), with and without high fat diet (HFD) challenge. Retinal microglia and macrophages were quantified by positive Iba1 and CD11b immunofluorescence.
Results: CD exposure caused impaired visual function and increased retinal immune cell expression in adult offspring. When challenged with HFD, CD offspring also exhibited altered retinal function and sex-specific impairments in glucose tolerance.
Conclusions: Overall, these findings suggest that the light environment contributes to developmental programming of the metabolic and visual systems, potentially promoting a pro-inflammatory milieu in the retina and increasing the risk of visual disease later in life.
Conflict of interest statement
Disclosure:
Figures






Similar articles
-
The impact of prenatal circadian rhythm disruption on pregnancy outcomes and long-term metabolic health of mice progeny.Chronobiol Int. 2016;33(9):1171-1181. doi: 10.1080/07420528.2016.1207661. Epub 2016 Jul 27. Chronobiol Int. 2016. PMID: 27463559
-
Circadian perinatal photoperiod has enduring effects on retinal dopamine and visual function.J Neurosci. 2014 Mar 26;34(13):4627-33. doi: 10.1523/JNEUROSCI.4887-13.2014. J Neurosci. 2014. PMID: 24672008 Free PMC article.
-
Pre-Weaning Exposure to Maternal High-Fat Diet Is a Critical Developmental Window for Programming the Metabolic System of Offspring in Mice.Front Endocrinol (Lausanne). 2022 Feb 10;13:816107. doi: 10.3389/fendo.2022.816107. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35222275 Free PMC article.
-
Light and Circadian Signaling Pathway in Pregnancy: Programming of Adult Health and Disease.Int J Mol Sci. 2020 Mar 23;21(6):2232. doi: 10.3390/ijms21062232. Int J Mol Sci. 2020. PMID: 32210175 Free PMC article. Review.
-
The Retina and Other Light-sensitive Ocular Clocks.J Biol Rhythms. 2016 Jun;31(3):223-43. doi: 10.1177/0748730416642657. Epub 2016 Apr 19. J Biol Rhythms. 2016. PMID: 27095816 Free PMC article. Review.
Cited by
-
A High Fat "Western-style" Diet Induces AMD-Like Features in Wildtype Mice.Mol Nutr Food Res. 2022 Jun;66(11):e2100823. doi: 10.1002/mnfr.202100823. Epub 2022 Apr 28. Mol Nutr Food Res. 2022. PMID: 35306732 Free PMC article.
-
Retinal and metabolic changes in a high-fat diet (HFD)+STZ model of Type II diabetes.Mol Vis. 2024 Jul 1;30:239-259. eCollection 2024. Mol Vis. 2024. PMID: 39959182 Free PMC article.
-
Developmental chronodisruption alters placental signaling in mice.PLoS One. 2021 Aug 9;16(8):e0255296. doi: 10.1371/journal.pone.0255296. eCollection 2021. PLoS One. 2021. PMID: 34370755 Free PMC article.
References
-
- Barker DJP. The developmental origins of adult disease. J Am Coll Nutr . 2004; 23(Suppl 6): 588S–595S. - PubMed
-
- McMillen IC, Robinson JS.. Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol Rev . 2005; 85(2): 571–633. - PubMed
-
- Varcoe TJ, Gatford KL, Kennaway DJ.. Maternal circadian rhythms and the programming of adult health and disease. Am J Physiol Regul Integr Comp Physiol . 2018; 314(2): R231–R241. - PubMed
-
- Fonken LK, Nelson RJ.. Effects of light exposure at night during development. Curr Opin Behav Sci . 2016; 7: 33–39.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

