Urea-Urease Reaction in Controlling Properties of Supramolecular Hydrogels: Pros and Cons
- PMID: 33861488
- PMCID: PMC8360084
- DOI: 10.1002/chem.202100490
Urea-Urease Reaction in Controlling Properties of Supramolecular Hydrogels: Pros and Cons
Abstract
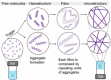
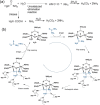
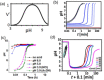
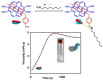
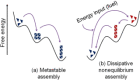
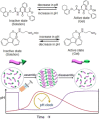
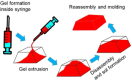
Supramolecular hydrogels are useful in many areas such as cell culturing, catalysis, sensing, tissue engineering, drug delivery, environmental remediation and optoelectronics. The gels need specific properties for each application. The properties arise from a fibrous network that forms the matrix. A common method to prepare hydrogels is to use a pH change. Most methods result in a sudden pH jump and often lead to gels that are hard to reproduce and control. The urease-urea reaction can be used to control hydrogel properties by a uniform and controlled pH increase as well as to set up pH cycles. The reaction involves hydrolysis of urea by urease and production of ammonia which increases the pH. The rate of ammonia production can be controlled which can be used to prepare gels with differing properties. Herein, we show how the urease-urea reaction can be used for the construction of next generation functional materials.
Keywords: Institute and/or researcher Twitter usernames: @prof_djadams; dynamic gels; hydrogels; kinetic control; pH-responsiveness; urease-urea reaction.
© 2021 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
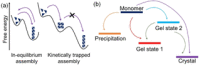



Similar articles
-
Enzymatic gelation to prepare chitosan gels: Study of gelation kinetics and identification of limiting parameters for controlled gel morphology.Int J Biol Macromol. 2018 Feb;107(Pt A):1175-1183. doi: 10.1016/j.ijbiomac.2017.09.097. Epub 2017 Sep 23. Int J Biol Macromol. 2018. PMID: 28951304
-
A determination and comparison of urease activity in feces and fresh manure from pig and cattle in relation to ammonia production and pH changes.PLoS One. 2014 Nov 14;9(11):e110402. doi: 10.1371/journal.pone.0110402. eCollection 2014. PLoS One. 2014. PMID: 25397404 Free PMC article.
-
Activity of urea amidohydrolase immobilized within poly[di(methoxyethoxyethoxy)phosphazene] hydrogels.Biomaterials. 1994 Jun;15(7):502-6. doi: 10.1016/0142-9612(94)90015-9. Biomaterials. 1994. PMID: 7918902
-
Maintaining homogeneity during a sol-gel transition by an autocatalytic enzyme reaction.Chem Commun (Camb). 2018 Dec 18;55(1):47-50. doi: 10.1039/c8cc08501c. Chem Commun (Camb). 2018. PMID: 30507994
-
Gradient Hydrogels-The State of the Art in Preparation Methods.Polymers (Basel). 2020 Apr 21;12(4):966. doi: 10.3390/polym12040966. Polymers (Basel). 2020. PMID: 32326192 Free PMC article. Review.
Cited by
-
Self-Regulating Hydrogel with Reversible Optical Activity in Its Gel-to-Gel Transformation.J Am Chem Soc. 2025 May 21;147(20):17361-17371. doi: 10.1021/jacs.5c03844. Epub 2025 May 9. J Am Chem Soc. 2025. PMID: 40344185 Free PMC article.
-
Chitosan Hydrogels Enriched with Biocompounds Extracted from Marine Sponges: Potential to Modulate the Inflammatory Process in an In Vitro Study.ACS Omega. 2025 Jun 12;10(24):25605-25620. doi: 10.1021/acsomega.5c01171. eCollection 2025 Jun 24. ACS Omega. 2025. PMID: 40584336 Free PMC article.
-
Understanding multicomponent low molecular weight gels from gelators to networks.J Adv Res. 2025 Mar;69:91-106. doi: 10.1016/j.jare.2024.03.028. Epub 2024 Apr 1. J Adv Res. 2025. PMID: 38570015 Free PMC article. Review.
-
Controlling the lifetime of cucurbit[8]uril based self-abolishing nanozymes.Chem Sci. 2022 Feb 14;13(14):4050-4057. doi: 10.1039/d1sc07203j. eCollection 2022 Apr 6. Chem Sci. 2022. PMID: 35440999 Free PMC article.
-
A Light Scattering Investigation of Enzymatic Gelation in Self-Assembling Peptides.Gels. 2023 Apr 19;9(4):347. doi: 10.3390/gels9040347. Gels. 2023. PMID: 37102959 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

