Podocyte Sphingolipid Signaling in Nephrotic Syndrome
- PMID: 33861526
- PMCID: PMC8193717
- DOI: 10.33594/000000356
Podocyte Sphingolipid Signaling in Nephrotic Syndrome
Abstract
Podocytes play a vital role in the pathogenesis of nephrotic syndrome (NS), which is clinically characterized by heavy proteinuria, hypoalbuminemia, hyperlipidemia, and peripheral edema. The pathogenesis of NS has evolved through several hypotheses ranging from immune dysregulation theory and increased glomerular permeability theory to the current concept of podocytopathy. Podocytopathy is characterized by dysfunction or depletion of podocytes, which may be caused by unknown permeability factor, genetic disorders, drugs, infections, systemic disorders, and hyperfiltration. Over the last two decades, numerous studies have been done to explore the molecular mechanisms of podocyte injuries or NS and to develop the novel therapeutic strategies targeting podocytopathy for treatment of NS. Recent studies have shown that normal sphingolipid metabolism is essential for structural and functional integrity of podocytes. As a basic component of the plasma membrane, sphingolipids not only support the assembly of signaling molecules and interaction of receptors and effectors, but also mediate various cellular activities, such as apoptosis, proliferation, stress responses, necrosis, inflammation, autophagy, senescence, and differentiation. This review briefly summarizes current evidence demonstrating the regulation of sphingolipid metabolism in podocytes and the canonical or noncanonical roles of podocyte sphingolipid signaling in the pathogenesis of NS and associated therapeutic strategies.
Keywords: Sphingolipid; Acid ceramidase; Sphingomyelin-like phosphodiesterase 3b; Podocyte; Nephrotic syndrome.
© Copyright by the Author(s). Published by Cell Physiol Biochem Press.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
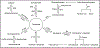


References
-
- Hannun YA, Obeid LM: The Ceramide-centric universe of lipid-mediated cell regulation: stress encounters of the lipid kind. J Biol Chem 2002;277:25847–25850. - PubMed
-
- Spiegel S, Milstien S: Sphingosine 1-phosphate, a key cell signaling molecule. J Biol Chem 2002;277:25851–25854. - PubMed
-
- Lavieu G, Scarlatti F, Sala G, Carpentier S, Levade T, Ghidoni R, Botti J, Codogno P: Regulation of autophagy by sphingosine kinase 1 and its role in cell survival during nutrient starvation. J Biol Chem 2006;281:8518–8527. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

