Pulmonary Arterial Hypertension: Diagnosis, Treatment, and Novel Advances
- PMID: 33861689
- PMCID: PMC8483220
- DOI: 10.1164/rccm.202012-4317SO
Pulmonary Arterial Hypertension: Diagnosis, Treatment, and Novel Advances
Abstract
The diagnosis and management of pulmonary arterial hypertension (PAH) includes several advances, such as a broader recognition of extrapulmonary vascular organ system involvement, validated point-of-care clinical assessment tools, and focus on the early initiation of multiple pharmacotherapeutics in appropriate patients. Indeed, a principal goal in PAH today is an early diagnosis for prompt initiation of treatment to achieve a minimal symptom burden; optimize the patient's biochemical, hemodynamic, and functional profile; and limit adverse events. To accomplish this end, clinicians must be familiar with novel risk factors and the revised hemodynamic definition for PAH. Fresh insights into the role of developmental biology (i.e., perinatal health) may also be useful for predicting incident PAH in early adulthood. Emergent or underused approaches to PAH management include a novel TGF-β ligand trap pharmacotherapy, remote pulmonary arterial pressure monitoring, next-generation imaging using inert gas-based magnetic resonance and other technologies, right atrial pacing, and pulmonary arterial denervation. These and other PAH state of the art advances are summarized here for the wider pulmonary medicine community.
Keywords: pulmonary arterial hypertension; pulmonary hypertension; risk stratification; treatment.
Figures
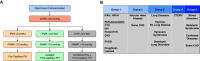

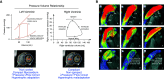
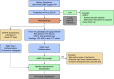




References
-
- Maron BA, Ryan JJ. A concerning trend for patients with pulmonary hypertension in the era of evidence-based medicine. Circulation. 2019;139:1861–1864. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 HL095686/HL/NHLBI NIH HHS/United States
- R01 HL158714/HL/NHLBI NIH HHS/United States
- R01 HL140731/HL/NHLBI NIH HHS/United States
- R01 HL127028/HL/NHLBI NIH HHS/United States
- R01 HL153502/HL/NHLBI NIH HHS/United States
- I01 BX005628/BX/BLRD VA/United States
- R01 HL149423/HL/NHLBI NIH HHS/United States
- R56 HL131787/HL/NHLBI NIH HHS/United States
- R01 HL122887/HL/NHLBI NIH HHS/United States
- R01 HL068702/HL/NHLBI NIH HHS/United States
- R01 HL138473/HL/NHLBI NIH HHS/United States
- R21 HL145420/HL/NHLBI NIH HHS/United States
- U54 HL119145/HL/NHLBI NIH HHS/United States
- R01 HL155096/HL/NHLBI NIH HHS/United States
- R01 HL141105/HL/NHLBI NIH HHS/United States
- R01 HL107577/HL/NHLBI NIH HHS/United States
- P01 HL014985/HL/NHLBI NIH HHS/United States
- R01 HL139613/HL/NHLBI NIH HHS/United States
- R01 HL145679/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

