Trichomonas vaginalis infection impairs anion secretion in vaginal epithelium
- PMID: 33861752
- PMCID: PMC8051796
- DOI: 10.1371/journal.pntd.0009319
Trichomonas vaginalis infection impairs anion secretion in vaginal epithelium
Abstract
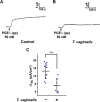
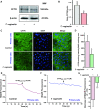
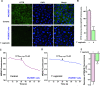
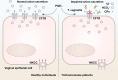
Trichomonas vaginalis is a common protozoan parasite, which causes trichomoniasis associated with severe adverse reproductive outcomes. However, the underlying pathogenesis has not been fully understood. As the first line of defense against invading pathogens, the vaginal epithelial cells are highly responsive to environmental stimuli and contribute to the formation of the optimal luminal fluid microenvironment. The cystic fibrosis transmembrane conductance regulator (CFTR), an anion channel widely distributed at the apical membrane of epithelial cells, plays a crucial role in mediating the secretion of Cl- and HCO3-. In this study, we investigated the effect of T. vaginalis on vaginal epithelial ion transport elicited by prostaglandin E2 (PGE2), a major prostaglandin in the semen. Luminal administration of PGE2 triggered a remarkable and sustained increase of short-circuit current (ISC) in rat vaginal epithelium, which was mainly due to Cl- and HCO3- secretion mediated by the cAMP-activated CFTR. However, T. vaginalis infection significantly abrogated the ISC response evoked by PGE2, indicating impaired transepithelial anion transport via CFTR. Using a primary cell culture system of rat vaginal epithelium and a human vaginal epithelial cell line, we demonstrated that the expression of CFTR was significantly down-regulated after T. vaginalis infection. In addition, defective Cl- transport function of CFTR was observed in T. vaginalis-infected cells by measuring intracellular Cl- signals. Conclusively, T. vaginalis restrained exogenous PGE2-induced anion secretion through down-regulation of CFTR in vaginal epithelium. These results provide novel insights into the intervention of reproductive complications associated with T. vaginalis infection such as infertility and disequilibrium in vaginal fluid microenvironment.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

