Validation of a new wearable device for type 3 sleep test without flowmeter
- PMID: 33861776
- PMCID: PMC8051765
- DOI: 10.1371/journal.pone.0249470
Validation of a new wearable device for type 3 sleep test without flowmeter
Abstract
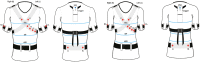
Background: Ventilation monitoring during sleep is performed by sleep test instrumentation that is uncomfortable for the patients due to the presence of the flowmeter. The objective of this study was to evaluate if an innovative type 3 wearable system, the X10X and X10Y, is able to correctly detect events of apnea and hypopnea and to classify the severity of sleep apnea without the use of a flowmeter.
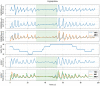
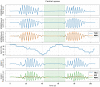
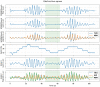
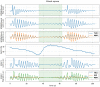
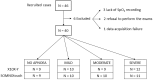
Methods: 40 patients with sleep disordered breathing were analyzed by continuous and simultaneous recording of X10X and X10Y and another certified type 3 system, SOMNOtouch, used for comparison. Evaluation was performed in terms of quality of respiratory signals (scores from 1, lowest, to 5, highest), duration and classification of apneas, as well as identification and duration of hypopneas.
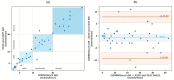
Results: 580 periods were evaluated. Mean quality assigned score was 3.37±1.42 and 3.25±1.35 for X10X and X10Y and SOMNOtouch, respectively. The agreement between the two systems was evaluated with grades 4 and 5 in 383 out of 580 cases. A high correlation (r2 = 0.921; p<0.001) was found between the AHI indexes obtained from the two systems. X10X and X10Y devices were able to correctly classify 72.3% of the obstructive apneas, 81% of the central apneas, 61.3% of the hypopneas, and 64.6% of the mixed apneas when compared to SOMNOtouch device.
Conclusion: The X10X and X10Y devices are able to provide a correct grading of sleep respiratory disorders without the need of a nasal cannula for respiratory flow measurement and can be considered as a type 3 sleep test device for screening tests.
Conflict of interest statement
Dr. Longinotti-Buitoni and Dr. Aliverti are inventors in patents related to the submitted work (Patents number US20200323285, US20200068708, US20180376586, US20180184735, US9282893). Dr. Aliverti received consultancy fees from L.I.F.E. Corporation S.A. outside the submitted work. Dr. Agostoni has non-financial support from Menarini, grants from Daiichi Sankyo, nonfinancial support from Novartis, non-financial support from Boeringer, grants and nonfinancial support from Actelion, and grants from Bayer, outside the work presented in this article. This does not alter the adherence to PLOS ONE policies on sharing data and materials.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

