Treatment profiles and clinical outcomes of COVID-19 patients at private hospital in Jakarta
- PMID: 33861777
- PMCID: PMC8051784
- DOI: 10.1371/journal.pone.0250147
Treatment profiles and clinical outcomes of COVID-19 patients at private hospital in Jakarta
Abstract
Background: Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) is a virus that causes COVID-19, which has become a worldwide pandemic. However, until now, there is no vaccine or specific drug to prevent or treat COVID-19.
Objectives: To find out the effective treatment as an antiviral agent for COVID-19, to determine the correlation between sociodemography with clinical outcomes and duration of treatment, and to determine the relationship between comorbidities with clinical outcomes and duration of treatment for COVID-19 patients.
Methods: A prospective cohort study was conducted in this study. This study included only confirmed COVID-19 patients who were admitted to the hospital during April-May 2020. Convenience sampling was used to select 103 patients, but only 72 patients were suitable for inclusion.
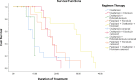
Results: The survival analysis for COVID-19 patients using the Kaplan Meier method showed that patients receiving Oseltamivir + Hydroxychloroquine had an average survival rate of about 83% after undergoing treatment of about ten days. Gender (p = 0.450) and age (p = 0.226) did not have a significant correlation with the duration of treatment for COVID-19 patients. Gender (p = 0.174) and age (p = 0.065) also did not have a significant correlation with clinical outcome of COVID-19 patients. Comorbidities showed a significant correlation with duration of treatment (p = 0.002) and clinical outcome (p = 0.014) of COVID-19 patients.
Conclusion: The most effective antiviral agent in this study based on treatment duration was the combination of Oseltamivir + Hydroxychloroquine. The higher the patient's average treatment duration is, the lower the average survival rate for COVID-19 patients.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Retrospective evaluation of seven different treatment protocols in hospitalized COVID-19 patients.Turk J Med Sci. 2021 Dec 13;51(6):2835-2849. doi: 10.3906/sag-2106-114. Turk J Med Sci. 2021. PMID: 34418000 Free PMC article.
-
Impact of cytokine storm on severity of COVID-19 disease in a private hospital in West Jakarta prior to vaccination.PLoS One. 2022 Jan 25;17(1):e0262438. doi: 10.1371/journal.pone.0262438. eCollection 2022. PLoS One. 2022. PMID: 35077495 Free PMC article.
-
Risk Factors Associated With In-Hospital Mortality in a US National Sample of Patients With COVID-19.JAMA Netw Open. 2020 Dec 1;3(12):e2029058. doi: 10.1001/jamanetworkopen.2020.29058. JAMA Netw Open. 2020. PMID: 33301018 Free PMC article.
-
Tracheotomy Outcomes in 64 Ventilated COVID-19 Patients at a High-Volume Center in Bronx, NY.Laryngoscope. 2021 Jun;131(6):E1797-E1804. doi: 10.1002/lary.29391. Epub 2021 Jan 27. Laryngoscope. 2021. PMID: 33410517
-
Questions about Tosepu et al. (2020) "Correlation between weather and Covid-19 pandemic in Jakarta, Indonesia".Sci Total Environ. 2022 Jun 15;825:154078. doi: 10.1016/j.scitotenv.2022.154078. Epub 2022 Feb 24. Sci Total Environ. 2022. PMID: 35219672 Free PMC article. Review.
Cited by
-
Comparison of Different Antiviral Regimens in the Treatment of Patients with Severe COVID-19: A Retrospective Cohort.Medicina (Kaunas). 2023 Jan 29;59(2):260. doi: 10.3390/medicina59020260. Medicina (Kaunas). 2023. PMID: 36837462 Free PMC article.
-
Time to Recovery from Covid-19 and Its Predictors Among Patients Admitted to Treatment Centers of Southern Nations Nationalities and Peoples Region (SNNPR), ETHIOPIA: Multi-Center Retrospective Cohort Study.Infect Drug Resist. 2022 Jun 16;15:3047-3062. doi: 10.2147/IDR.S365986. eCollection 2022. Infect Drug Resist. 2022. PMID: 35747331 Free PMC article.
-
A validated UHPLC-MS/MS method for simultaneous quantification of some repurposed COVID-19 drugs in rat plasma: Application to a pharmacokinetic study.Microchem J. 2022 Jul;178:107321. doi: 10.1016/j.microc.2022.107321. Epub 2022 Mar 3. Microchem J. 2022. PMID: 35261396 Free PMC article.
-
Infectious Disease Modeling with Socio-Viral Behavioral Aspects-Lessons Learned from the Spread of SARS-CoV-2 in a University.Trop Med Infect Dis. 2022 Oct 9;7(10):289. doi: 10.3390/tropicalmed7100289. Trop Med Infect Dis. 2022. PMID: 36288030 Free PMC article.
-
Clinical profile and outcomes of COVID-19 positive patients -A cross sectional study.J Family Med Prim Care. 2021 Nov;10(11):4036-4040. doi: 10.4103/jfmpc.jfmpc_301_21. Epub 2021 Nov 29. J Family Med Prim Care. 2021. PMID: 35136764 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous