Nuts and bolts of the salt-inducible kinases (SIKs)
- PMID: 33861845
- PMCID: PMC8057676
- DOI: 10.1042/BCJ20200502
Nuts and bolts of the salt-inducible kinases (SIKs)
Abstract
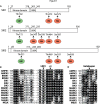
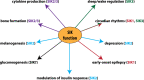
The salt-inducible kinases, SIK1, SIK2 and SIK3, most closely resemble the AMP-activated protein kinase (AMPK) and other AMPK-related kinases, and like these family members they require phosphorylation by LKB1 to be catalytically active. However, unlike other AMPK-related kinases they are phosphorylated by cyclic AMP-dependent protein kinase (PKA), which promotes their binding to 14-3-3 proteins and inactivation. The most well-established substrates of the SIKs are the CREB-regulated transcriptional co-activators (CRTCs), and the Class 2a histone deacetylases (HDAC4/5/7/9). Phosphorylation by SIKs promotes the translocation of CRTCs and Class 2a HDACs to the cytoplasm and their binding to 14-3-3s, preventing them from regulating their nuclear binding partners, the transcription factors CREB and MEF2. This process is reversed by PKA-dependent inactivation of the SIKs leading to dephosphorylation of CRTCs and Class 2a HDACs and their re-entry into the nucleus. Through the reversible regulation of these substrates and others that have not yet been identified, the SIKs regulate many physiological processes ranging from innate immunity, circadian rhythms and bone formation, to skin pigmentation and metabolism. This review summarises current knowledge of the SIKs and the evidence underpinning these findings, and discusses the therapeutic potential of SIK inhibitors for the treatment of disease.
Keywords: AMPK-related kinase; CREB; CREB-regulated transcriptional co-activator (CRTC); histone deacetylase (HDAC); myocyte enhancer factor 2 (MEF2); salt-inducible kinase (SIK).
© 2021 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

