Validation of a host blood transcriptomic biomarker for pulmonary tuberculosis in people living with HIV: a prospective diagnostic and prognostic accuracy study
- PMID: 33862012
- PMCID: PMC8131200
- DOI: 10.1016/S2214-109X(21)00045-0
Validation of a host blood transcriptomic biomarker for pulmonary tuberculosis in people living with HIV: a prospective diagnostic and prognostic accuracy study
Abstract
Background: A rapid, blood-based triage test that allows targeted investigation for tuberculosis at the point of care could shorten the time to tuberculosis treatment and reduce mortality. We aimed to test the performance of a host blood transcriptomic signature (RISK11) in diagnosing tuberculosis and predicting progression to active pulmonary disease (prognosis) in people with HIV in a community setting.
Methods: In this prospective diagnostic and prognostic accuracy study, adults (aged 18-59 years) with HIV were recruited from five communities in South Africa. Individuals with a history of tuberculosis or household exposure to multidrug-resistant tuberculosis within the past 3 years, comorbid risk factors for tuberculosis, or any condition that would interfere with the study were excluded. RISK11 status was assessed at baseline by real-time PCR; participants and study staff were masked to the result. Participants underwent active surveillance for microbiologically confirmed tuberculosis by providing spontaneously expectorated sputum samples at baseline, if symptomatic during 15 months of follow-up, and at 15 months (the end of the study). The coprimary outcomes were the prevalence and cumulative incidence of tuberculosis disease confirmed by a positive Xpert MTB/RIF, Xpert Ultra, or Mycobacteria Growth Indicator Tube culture, or a combination of such, on at least two separate sputum samples collected within any 30-day period.
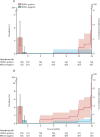
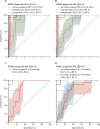
Findings: Between March 22, 2017, and May 15, 2018, 963 participants were assessed for eligibility and 861 were enrolled. Among 820 participants with valid RISK11 results, eight (1%) had prevalent tuberculosis at baseline: seven (2·5%; 95% CI 1·2-5·0) of 285 RISK11-positive participants and one (0·2%; 0·0-1·1) of 535 RISK11-negative participants. The relative risk (RR) of prevalent tuberculosis was 13·1 times (95% CI 2·1-81·6) greater in RISK11-positive participants than in RISK11-negative participants. RISK11 had a diagnostic area under the receiver operating characteristic curve (AUC) of 88·2% (95% CI 77·6-96·7), and a sensitivity of 87·5% (58·3-100·0) and specificity of 65·8% (62·5-69·0) at a predefined score threshold (60%). Of those with RISK11 results, eight had primary endpoint incident tuberculosis during 15 months of follow-up. Tuberculosis incidence was 2·5 per 100 person-years (95% CI 0·7-4·4) in the RISK11-positive group and 0·2 per 100 person-years (0·0-0·5) in the RISK11-negative group. The probability of primary endpoint incident tuberculosis was greater in the RISK11-positive group than in the RISK11-negative group (cumulative incidence ratio 16·0 [95% CI 2·0-129·5]). RISK11 had a prognostic AUC of 80·0% (95% CI 70·6-86·9), and a sensitivity of 88·6% (43·5-98·7) and a specificity of 68·9% (65·3-72·3) for incident tuberculosis at the 60% threshold.
Interpretation: RISK11 identified prevalent tuberculosis and predicted risk of progression to incident tuberculosis within 15 months in ambulant people living with HIV. RISK11's performance approached, but did not meet, WHO's target product profile benchmarks for screening and prognostic tests for tuberculosis.
Funding: Bill & Melinda Gates Foundation and the South African Medical Research Council.
Copyright © 2021 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests AP-N, GW, GC, TJS, and MH report grants from the Bill & Melinda Gates Foundation during the conduct of the study. AP-N and GW report grants from the South African Medical Research Council during the conduct of the study. GW and TJS report grants from the South African National Research Foundation during the conduct of the study. AP-N and TJS have patents of the RISK11 and RISK6 signatures pending. GW has had a patent (tuberculosis diagnostic markers; PCT/IB2013/054377) issued and a patent (method for diagnosing tuberculosis; PCT/IB2017/052142) pending. All other authors declare no competing interests.
Figures


Comment in
-
Validating novel diagnostic assays for tuberculosis in the context of existing tools.Lancet Glob Health. 2021 Sep;9(9):e1209. doi: 10.1016/S2214-109X(21)00306-5. Lancet Glob Health. 2021. PMID: 34416206 Free PMC article. No abstract available.
References
-
- WHO The End TB Strategy. Global strategy and targets for tuberculosis prevention, care and control after 2015. 2015. https://www.who.int/tb/strategy/End_TB_Strategy.pdf?ua=1
-
- Hamada Y, Lujan J, Schenkel K, Ford N, Getahun H. Sensitivity and specificity of WHO's recommended four-symptom screening rule for tuberculosis in people living with HIV: a systematic review and meta-analysis. Lancet HIV. 2018;5:e515–e523. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

