Human challenge study with a Shigella bioconjugate vaccine: Analyses of clinical efficacy and correlate of protection
- PMID: 33862589
- PMCID: PMC8054157
- DOI: 10.1016/j.ebiom.2021.103310
Human challenge study with a Shigella bioconjugate vaccine: Analyses of clinical efficacy and correlate of protection
Abstract
Background: Shigellosis is a major cause of moderate to severe diarrhoea and dysentery in children under 5 years of age in low and middle-income countries. The Flexyn2a vaccine conjugates the O-polysaccharide of Shigella flexneri 2a to Pseudomonas aeruginosa exotoxin A. We describe a Phase 2b proof-of-concept challenge study that evaluated safety, immunogenicity, and efficacy of the Flexyn2a vaccine to protect against shigellosis.
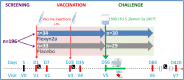
Methods: In this randomized, double blind, placebo-controlled trial, healthy adults were randomized 1:1 to receive Flexyn2a (10 µg) or placebo intramuscularly, twice, 4 weeks apart, followed by challenge 4 weeks later with 1500 colony forming units (CFUs) of S. flexneri 2a strain 2457T. The primary outcome was vaccine-induced protection. S. flexneri 2a lipopolysaccharide (LPS)-specific immune responses were assessed.
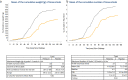
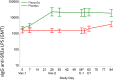
Findings: Sixty-seven subjects were enrolled, 34 received vaccine and 33 placebo. The vaccine was well tolerated; the majority of adverse events were mild in nature. Thirty vaccinees and 29 placebo recipients received the S. flexneri 2a challenge. Vaccination resulted in a 30.2% reduction in shigellosis compared with placebo (13/30 vs. 18/29; p = 0.11; 95% CI -15 to 62.6). Vaccine efficacy was more robust against severe disease, reaching 51.7% (p = 0.015, 95% CI 5.3 to 77.9) against moderate/severe diarrhoea or dysentery concurrent with fever or severe enteric symptoms and 72.4% (p = 0.07) against more severe diarrhoea (≥10 lose stools or ≥1000 g loose stools/24 h). Vaccinated subjects were less likely to need early antibiotic intervention following challenge (protective efficacy 51.7%, p = 0.01; 95% CI 9 to 76.8). In those who developed shigellosis, vaccinated subjects had a lower disease severity score (p = 0.002) than placebo-recipients. Additionally, LPS-specific serum IgG responses in Flexyn2a recipients were associated with protection against disease (p = 0.0016) and with a decreased shigellosis disease score (p = 0.002).
Interpretation: The Flexyn2a bioconjugate vaccine was immunogenic, well tolerated and protected against severe illness after Shigella challenge and is a promising Shigella vaccine construct. We identified a strong association between anti-S. flexneri 2a serum IgG and a reduction in disease outcomes. (Clinicaltrials.gov, NCT02646371.) FUNDING: Funding for this study was through a grant from the Wellcome Trust.
Keywords: Bioconjugate vaccine; Controlled human challenge study; Shigella; Shigella flexneri 2a; Vaccine.
Copyright © 2021 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest CA, PM, AMD, RF and VGF were employees of LimmaTech Biologics at the time of the study. KRT, CA, PM, ALB, AMD, RWK, SC, KAC, JB, RF, BD, HW, BF, JH, DS, VGF received grant support from the Wellcome Trust. The authors declare no other competing interests.
Figures



Comment in
-
Shigella conjugate vaccine efficacy trial in controlled human model and potential immune correlates of protection.EBioMedicine. 2021 Apr;66:103343. doi: 10.1016/j.ebiom.2021.103343. Epub 2021 Apr 16. EBioMedicine. 2021. PMID: 33873142 Free PMC article. No abstract available.
References
-
- Black R.E., Brown K.H., Becker S. Effects of diarrhea associated with specific enteropathogens on the growth of children in rural Bangladesh. Pediatrics. 1984;73(6):799–805. - PubMed
-
- Lee G., Paredes Olortegui M., Yori P.P. Effects of Shigella, campylobacter- and ETEC-associated diarrhea on childhood growth. Pediatr Infect Dis J. 2014;33(10):1004–1009. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

