In Situ Programming of CAR T Cells
- PMID: 33863239
- PMCID: PMC9007322
- DOI: 10.1146/annurev-bioeng-070620-033348
In Situ Programming of CAR T Cells
Abstract
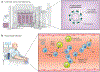
Gene therapy makes it possible to engineer chimeric antigen receptors (CARs) to create T cells that target specific diseases. However, current approaches require elaborate and expensive protocols to manufacture engineered T cells ex vivo, putting this therapy beyond the reach of many patients who might benefit. A solution could be to program T cells in vivo. Here, we evaluate the clinical need for in situ CAR T cell programming, compare competing technologies, review current progress, and provide a perspective on the long-term impact of this emerging and rapidly flourishing biotechnology field.
Keywords: CAR T cell therapy; gene therapy; nanoparticle; off-the-shelf T cell therapy.
Conflict of interest statement
DISCLOSURE STATEMENT
M.T.S. is a consultant of Tidal Therapeutics and holds stocks in the company. N.N.P. declares no competing interest.
Figures


References
-
- Rockoff JD. 2018. The million-dollar cancer treatment: Who will pay? Wall Street J. Apr. 26. https://www.wsj.com/articles/the-million-dollar-cancer-treatment-no-one-...
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

