Comparative efficacy of subcutaneous (CT-P13) and intravenous infliximab in adult patients with rheumatoid arthritis: a network meta-regression of individual patient data from two randomised trials
- PMID: 33863352
- PMCID: PMC8051052
- DOI: 10.1186/s13075-021-02487-x
Comparative efficacy of subcutaneous (CT-P13) and intravenous infliximab in adult patients with rheumatoid arthritis: a network meta-regression of individual patient data from two randomised trials
Abstract
Background: A subcutaneous (SC) formulation of infliximab biosimilar CT-P13 is approved in Europe for the treatment of adult patients with rheumatoid arthritis (RA). It may offer improved efficacy versus intravenous (IV) infliximab formulations.
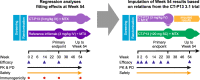
Methods: A network meta-regression was conducted using individual patient data from two randomised trials in patients with RA, which compared CT-P13 SC with CT-P13 IV, and CT-P13 IV with reference infliximab IV. In this analysis, CT-P13 SC was compared with CT-P13 IV, reference infliximab IV and pooled data for both reference infliximab IV and CT-P13 IV. Outcomes included changes from baseline in 28-joint Disease Activity Score based on C-reactive protein (DAS28-CRP), Simplified Disease Activity Index (SDAI) and Clinical Disease Activity Index (CDAI), and rates of remission, low disease activity or clinically meaningful improvement in functional disability per Health Assessment Questionnaire-Disability Index (HAQ-DI).
Results: The two studies enrolled 949 patients with RA; pooled data for 840 and 751 patients were evaluable at weeks 30 and 54, respectively. For the CT-P13 SC versus pooled IV treatment arm comparison, differences in changes from baseline in DAS28-CRP (- 0.578; 95% confidence interval [CI] - 0.831, - 0.325; p < 0.0001), CDAI (- 3.502; 95% CI - 5.715, - 1.289; p = 0.002) and SDAI (- 4.031; 95% CI - 6.385, - 1.677; p = 0.0008) scores at 30 weeks were statistically significant in favour of CT-P13 SC. From weeks 30 to 54, the magnitude of the differences increased and remained statistically significant in favour of CT-P13 SC. Similar results were observed for the comparison of CT-P13 SC with CT-P13 IV and with reference infliximab IV. Statistically significant differences at week 30 favoured CT-P13 SC over the pooled IV treatment arms for the proportions of patients achieving EULAR-CRP good response, American College of Rheumatology (ACR) 50 and ACR70 responses, DAS28-CRP-defined remission, low disease activity (DAS28-CRP, CDAI and SDAI criteria) and clinically meaningful HAQ-DI improvement.
Conclusions: CT-P13 SC was associated with greater improvements in DAS28-CRP, CDAI and SDAI scores and higher rates of clinical response, low disease activity and clinically meaningful improvement in functional disability, compared with CT-P13 IV and reference infliximab IV.
Trial registration: EudraCT, 2016-002125-11 , registered 1 July 2016; EudraCT 2010-018646-31 , registered 23 June 2010.
Keywords: CT-P13; Disease activity; Indirect treatment comparison; Individual patient data; Infliximab; Intravenous; Network meta-regression; Rheumatoid arthritis; Subcutaneous; Tumour necrosis factor inhibitor.
Conflict of interest statement
BC received honoraria from AbbVie, Bristol-Myers Squibb, Gilead, Janssen, Lilly, Merck, Novartis, Pfizer, Roche-Chugai, Sanofi and UCB; and research grants from Novartis, Pfizer and Roche. YA declares no competing interests. RA received honoraria from AbbVie, Bristol-Myers Squibb, Celltrion, Gilead, Janssen, Lilly, Merck, Novartis, Pfizer, Roche-Chugai and UCB; and research grants from Novartis, Pfizer and Roche. RC received a speaker’s fee and a consultation grant from AbbVie, BMS, Celltrion, Fresenius-Kabi, Gilead-Galapagos, Lilly, MSD, Pfizer, Roche, Samsung-Bioepis, Sanofi and UCB. PD received speaker’s fees from AbbVie, Bristol-Myers Squibb, Galapagos, Lilly and Sanofi
Figures

Similar articles
-
A phase III randomized study to evaluate the efficacy and safety of CT-P13 compared with reference infliximab in patients with active rheumatoid arthritis: 54-week results from the PLANETRA study.Arthritis Res Ther. 2016 Apr 2;18:82. doi: 10.1186/s13075-016-0981-6. Arthritis Res Ther. 2016. PMID: 27038608 Free PMC article. Clinical Trial.
-
A randomised, double-blind, parallel-group study to demonstrate equivalence in efficacy and safety of CT-P13 compared with innovator infliximab when coadministered with methotrexate in patients with active rheumatoid arthritis: the PLANETRA study.Ann Rheum Dis. 2013 Oct;72(10):1613-20. doi: 10.1136/annrheumdis-2012-203090. Epub 2013 May 16. Ann Rheum Dis. 2013. PMID: 23687260 Free PMC article. Clinical Trial.
-
Retention Rate and Safety of Biosimilar CT-P13 in Rheumatoid Arthritis: Data from the Korean College of Rheumatology Biologics Registry.BioDrugs. 2020 Feb;34(1):89-98. doi: 10.1007/s40259-019-00393-y. BioDrugs. 2020. PMID: 31734899 Free PMC article.
-
Efficacy and safety of subcutaneous infliximab versus adalimumab, etanercept and intravenous infliximab in patients with rheumatoid arthritis: a systematic literature review and meta-analysis.Expert Rev Clin Immunol. 2021 Jan;17(1):85-99. doi: 10.1080/1744666X.2020.1858803. Epub 2020 Dec 28. Expert Rev Clin Immunol. 2021. PMID: 33305638
-
Subcutaneous Infliximab, CT-P13 SC: A Profile of Its Use in the EU.Clin Drug Investig. 2021 Dec;41(12):1099-1107. doi: 10.1007/s40261-021-01093-8. Epub 2021 Nov 2. Clin Drug Investig. 2021. PMID: 34727347 Review.
Cited by
-
Demographic and Clinical Characteristics of Patients with Sustained and Switching Treatments Using Biological and Targeted Synthetic Disease-Modifying Antirheumatic Drugs: A Multicenter, Observational Cross-Sectional Study for Rheumatoid Arthritis.Rheumatol Ther. 2022 Feb;9(1):223-241. doi: 10.1007/s40744-021-00403-y. Epub 2021 Dec 1. Rheumatol Ther. 2022. PMID: 34850376 Free PMC article.
-
Advancement in nanotechnology for treatment of rheumatoid arthritis: scope and potential applications.Naunyn Schmiedebergs Arch Pharmacol. 2023 Oct;396(10):2287-2310. doi: 10.1007/s00210-023-02514-5. Epub 2023 May 11. Naunyn Schmiedebergs Arch Pharmacol. 2023. PMID: 37166463 Review.
-
Translation Research in Therapeutic Approaches from Conventional to Novel Nano-therapeutics for Rheumatoid Arthritis Treatment.Curr Rheumatol Rev. 2025;21(1):37-53. doi: 10.2174/0115733971288433240408062359. Curr Rheumatol Rev. 2025. PMID: 38629371 Review.
-
Therapeutic Drug Monitoring of Subcutaneous Infliximab in Inflammatory Bowel Disease-Understanding Pharmacokinetics and Exposure Response Relationships in a New Era of Subcutaneous Biologics.J Clin Med. 2022 Oct 19;11(20):6173. doi: 10.3390/jcm11206173. J Clin Med. 2022. PMID: 36294494 Free PMC article. Review.
-
Re-Routing Infliximab Therapy: Subcutaneous Infliximab Opens a Path Towards Greater Convenience and Clinical Benefit.Clin Drug Investig. 2022 Jun;42(6):477-489. doi: 10.1007/s40261-022-01162-6. Epub 2022 Jun 3. Clin Drug Investig. 2022. PMID: 35657560 Review.
References
-
- Safiri S, Kolahi AA, Hoy D, Smith E, Bettampadi D, Mansournia MA, Almasi-Hashiani A, Ashrafi-Asgarabad A, Moradi-Lakeh M, Qorbani M, Collins G, Woolf AD, March L, Cross M. Global, regional and national burden of rheumatoid arthritis 1990–2017: a systematic analysis of the Global Burden of Disease study 2017. Ann Rheum Dis. 2019;78(11):1463–1471. doi: 10.1136/annrheumdis-2019-215920. - DOI - PubMed
-
- Centers for Disease Control and Prevention. Rheumatoid arthritis (RA). Available from: https://www.cdc.gov/arthritis/basics/rheumatoid-arthritis.html. [cited 2021 January 29].
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

