Toll-like receptor 2 induced senescence in intervertebral disc cells of patients with back pain can be attenuated by o-vanillin
- PMID: 33863359
- PMCID: PMC8051055
- DOI: 10.1186/s13075-021-02504-z
Toll-like receptor 2 induced senescence in intervertebral disc cells of patients with back pain can be attenuated by o-vanillin
Abstract
Background: There is an increased level of senescent cells and toll-like teceptor-1, -2, -4, and -6 (TLR) expression in degenerating intervertebral discs (IVDs) from back pain patients. However, it is currently not known if the increase in expression of TLRs is related to the senescent cells or if it is a more general increase on all cells. It is also not known if TLR activation in IVD cells will induce cell senescence.
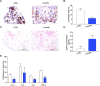
Methods: Cells from non-degenerate human IVD were obtained from spine donors and cells from degenerate IVDs came from patients undergoing surgery for low back pain. Gene expression of TLR-1,2,4,6, senescence and senescence-associated secretory phenotype (SASP) markers was evaluated by RT-qPCR in isolated cells. Matrix synthesis was verified with safranin-O staining and Dimethyl-Methylene Blue Assay (DMMB) confirmed proteoglycan content. Protein expression of p16INK4a, SASP factors, and TLR-2 was evaluated by immunocytochemistry (ICC) and/or by enzyme-linked immunosorbent assay (ELISA).
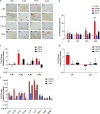
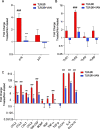
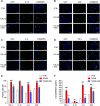
Results: An increase in senescent cells was found following 48-h induction with a TLR-2/6 agonist in cells from both non-degenerate and degenerating human IVDs. Higher levels of SASP factors, TLR-2 gene expression, and protein expression were found following 48-h induction with TLR-2/6 agonist. Treatment with o-vanillin reduced the number of senescent cells, and increased matrix synthesis in IVD cells from back pain patients. Treatment with o-vanillin after induction with TLR-2/6 agonist reduced gene and protein expression of SASP factors and TLR-2. Co-localized staining of p16INK4a and TLR-2 demonstrated that senescent cells have a high TLR-2 expression.
Conclusions: Taken together our data demonstrate that activation of TLR-2/6 induce senescence and increase TLR-2 and SASP expression in cells from non-degenerate IVDs of organ donors without degeneration and back pain and in cells from degenerating human IVD of patients with disc degeneration and back pain. The senescent cells showed high TLR-2 expression suggesting a link between TLR activation and cell senescence in human IVD cells. The reduction in senescence, SASP, and TLR-2 expression suggest o-vanillin as a potential disease-modifying drug for patients with disc degeneration and back pain.
Keywords: Back pain; Degeneration; Inflammation; Intervertebral disc; Senescence; Senolytics; Toll-like receptor 2; o-Vanillin.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Feng H, Danfelter M, Strömqvist B, Heinegård D. Extracellular matrix in disc degeneration. J Bone Joint Surg Am. 2006;88(Suppl 2):25–29. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

