Molecular Dissection of Perithecial Mating Line Development in Colletotrichum fructicola, a Species with a Nontypical Mating System Featuring Plus-to-Minus Switch and Plus-Minus-Mediated Sexual Enhancement
- PMID: 33863706
- PMCID: PMC8284469
- DOI: 10.1128/AEM.00474-21
Molecular Dissection of Perithecial Mating Line Development in Colletotrichum fructicola, a Species with a Nontypical Mating System Featuring Plus-to-Minus Switch and Plus-Minus-Mediated Sexual Enhancement
Abstract
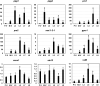
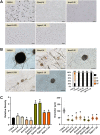
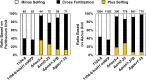
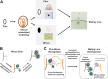
The genetic regulation of Colletotrichum (Glomerella) sexual reproduction does not strictly adhere to the Ascomycota paradigm and remains poorly understood. Morphologically different but sexually compatible strain types, termed plus and minus, have been recognized, but the biological and molecular distinctions between these strain types remain elusive. In this study, we characterized the sexual behaviors of a pair of plus and minus strains of C. fructicola with the aid of live-cell nucleus-localized fluorescent protein labeling, gene expression, and gene mutation analyses. We confirmed a genetically stable plus-to-minus switching phenomenon and demonstrated the presence of both cross-fertilized and self-fertilized perithecia within the mating line (perithecia cluster at the line of colony contact) between plus and minus strains. We demonstrated that pheromone signaling genes (a-factor-like and α-factor-like pheromones and their corresponding GPCR receptors) were differently expressed between vegetative hyphae of the two strains. Moreover, deletion of pmk1 (a FUS/KSS1 mitogen-activate protein kinase) in the minus strain severely limited mating line formation, whereas deletion of a GPCR (FGSG_05239 homolog) and two histone modification factors (hos2, snt2) in the minus strain did not affect mating line development but altered the ratio between cross-fertilization and self-fertilization within the mating line. We propose a model in which mating line formation in C. fructicola involves enhanced protoperithecium differentiation and enhanced perithecium maturation of the minus strain mediated by both cross-fertilization and diffusive effectors. This study provides insights into mechanisms underlying the mysterious phenomenon of plus-minus-mediated sexual enhancement being unique to Colletotrichum fungi. IMPORTANCE Plus-minus regulation of Colletotrichum sexual differentiation was reported in the early 1900s. Both plus and minus strains produce fertile perithecia in a homothallic but inefficient manner. However, when the two strain types encounter each other, efficient differentiation of fertile perithecia is triggered. The plus strain, by itself, can also generate minus ascospore progeny at high frequency. This nontypical mating system facilitates sexual reproduction and is Colletotrichum specific; the underlying molecular mechanisms, however, remain elusive. The current study revisits this longstanding mystery using C. fructicola as an experimental system. The presence of both cross-fertilized and self-fertilized perithecia within the mating line was directly evidenced by live-cell imaging with fluorescent markers. Based on further gene expression and gene mutation analysis, a model explaining mating line development (plus-minus-mediated sexual enhancement) is proposed. Data reported here have the potential to allow us to better understand Colletotrichum mating and filamentous ascomycete sexual regulation.
Keywords: Colletotrichum; mating type; perithecium; sexual reproduction.
Figures



Similar articles
-
Unique patterns of mating pheromone presence and absence could result in the ambiguous sexual behaviors of Colletotrichum species.G3 (Bethesda). 2021 Sep 6;11(9):jkab187. doi: 10.1093/g3journal/jkab187. G3 (Bethesda). 2021. PMID: 34544120 Free PMC article.
-
Genetic Networks That Govern Sexual Reproduction in the Pezizomycotina.Microbiol Mol Biol Rev. 2021 Dec 15;85(4):e0002021. doi: 10.1128/MMBR.00020-21. Epub 2021 Sep 29. Microbiol Mol Biol Rev. 2021. PMID: 34585983 Free PMC article. Review.
-
Two Putative Pheromone Receptors, but Not Their Cognate Pheromones, Regulate Female Fertility in the Atypical Mating Fungus Colletotrichum fructicola.Phytopathology. 2023 Oct;113(10):1934-1945. doi: 10.1094/PHYTO-11-22-0436-R. Epub 2023 Nov 10. Phytopathology. 2023. PMID: 37141175
-
CfCpmd1 Regulates Pathogenicity and Sexual Development of Plus and Minus Strains in Colletotrichum fructicola Causing Glomerella Leaf Spot on Apple in China.Phytopathology. 2023 Oct;113(10):1985-1993. doi: 10.1094/PHYTO-02-23-0071-R. Epub 2023 Nov 8. Phytopathology. 2023. PMID: 37129259
-
Cell and nuclear recognition mechanisms mediated by mating type in filamentous ascomycetes.Curr Opin Microbiol. 2000 Apr;3(2):183-8. doi: 10.1016/s1369-5274(00)00073-4. Curr Opin Microbiol. 2000. PMID: 10744990 Review.
Cited by
-
CfHMG Differentially Regulates the Sexual Development and Pathogenicity of Colletotrichum fructicola Plus and Minus Strains.J Fungi (Basel). 2024 Jul 11;10(7):478. doi: 10.3390/jof10070478. J Fungi (Basel). 2024. PMID: 39057363 Free PMC article.
-
Unique patterns of mating pheromone presence and absence could result in the ambiguous sexual behaviors of Colletotrichum species.G3 (Bethesda). 2021 Sep 6;11(9):jkab187. doi: 10.1093/g3journal/jkab187. G3 (Bethesda). 2021. PMID: 34544120 Free PMC article.
-
Genetic Networks That Govern Sexual Reproduction in the Pezizomycotina.Microbiol Mol Biol Rev. 2021 Dec 15;85(4):e0002021. doi: 10.1128/MMBR.00020-21. Epub 2021 Sep 29. Microbiol Mol Biol Rev. 2021. PMID: 34585983 Free PMC article. Review.
-
Population Genomics Provide Insights into the Global Genetic Structure of Colletotrichum graminicola, the Causal Agent of Maize Anthracnose.mBio. 2023 Feb 28;14(1):e0287822. doi: 10.1128/mbio.02878-22. Epub 2022 Dec 19. mBio. 2023. PMID: 36533926 Free PMC article.
-
Structure and number of mating pheromone genes is closely linked to sexual reproductive strategy in Huntiella.BMC Genomics. 2023 May 13;24(1):261. doi: 10.1186/s12864-023-09355-9. BMC Genomics. 2023. PMID: 37179314 Free PMC article.
References
-
- Crouch J, O’Connell R, Gan P, Buiate E, Torres MF, Beirn L, Shirasu K, Vaillancourt L. 2014. The genomics of Colletotrichum, p 69–102. In Dean AR, Lichens-Park A, Kole C (ed), Genomics of plant-associated fungi: monocot pathogens. Springer Press, Berlin, Germany.
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources

