Pyruvate Production by Escherichia coli by Use of Pyruvate Dehydrogenase Variants
- PMID: 33863707
- PMCID: PMC8315933
- DOI: 10.1128/AEM.00487-21
Pyruvate Production by Escherichia coli by Use of Pyruvate Dehydrogenase Variants
Abstract
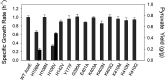
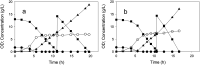
Altering metabolic flux at a key branch point in metabolism has commonly been accomplished through gene knockouts or by modulating gene expression. An alternative approach to direct metabolic flux preferentially toward a product is decreasing the activity of a key enzyme through protein engineering. In Escherichia coli, pyruvate can accumulate from glucose when carbon flux through the pyruvate dehydrogenase complex is suppressed. Based on this principle, 16 chromosomally expressed AceE variants were constructed in E. coli C and compared for growth rate and pyruvate accumulation using glucose as the sole carbon source. To prevent conversion of pyruvate to other products, the strains also contained deletions in two nonessential pathways: lactate dehydrogenase (ldhA) and pyruvate oxidase (poxB). The effect of deleting phosphoenolpyruvate synthase (ppsA) on pyruvate assimilation was also examined. The best pyruvate-accumulating strains were examined in controlled batch and continuous processes. In a nitrogen-limited chemostat process at steady-state growth rates of 0.15 to 0.28 h-1, an engineered strain expressing the AceE[H106V] variant accumulated pyruvate at a yield of 0.59 to 0.66 g pyruvate/g glucose with a specific productivity of 0.78 to 0.92 g pyruvate/g cells·h. These results provide proof of concept that pyruvate dehydrogenase complex variants can effectively shift carbon flux away from central carbon metabolism to allow pyruvate accumulation. This approach can potentially be applied to other key enzymes in metabolism to direct carbon toward a biochemical product. IMPORTANCE Microbial production of biochemicals from renewable resources has become an efficient and cost-effective alternative to traditional chemical synthesis methods. Metabolic engineering tools are important for optimizing a process to perform at an economically feasible level. This study describes an additional tool to modify central metabolism and direct metabolic flux to a product. We have shown that variants of the pyruvate dehydrogenase complex can direct metabolic flux away from cell growth to increase pyruvate production in Escherichia coli. This approach could be paired with existing strategies to optimize metabolism and create industrially relevant and economically feasible processes.
Keywords: batch; chemostat; fermentation; point mutation; pyruvic acid.
Figures


Similar articles
-
Conversion of glycerol to pyruvate by Escherichia coli using acetate- and acetate/glucose-limited fed-batch processes.J Ind Microbiol Biotechnol. 2010 Mar;37(3):307-12. doi: 10.1007/s10295-009-0675-z. Epub 2009 Dec 13. J Ind Microbiol Biotechnol. 2010. PMID: 20012884
-
Metabolic flux control at the pyruvate node in an anaerobic Escherichia coli strain with an active pyruvate dehydrogenase.Appl Environ Microbiol. 2010 Apr;76(7):2107-14. doi: 10.1128/AEM.02545-09. Epub 2010 Jan 29. Appl Environ Microbiol. 2010. PMID: 20118372 Free PMC article.
-
Citrate synthase variants improve yield of acetyl-CoA derived 3-hydroxybutyrate in Escherichia coli.Microb Cell Fact. 2024 Jun 12;23(1):173. doi: 10.1186/s12934-024-02444-8. Microb Cell Fact. 2024. PMID: 38867236 Free PMC article.
-
Alteration of the biochemical valves in the central metabolism of Escherichia coli.Ann N Y Acad Sci. 1994 Nov 30;745:21-34. doi: 10.1111/j.1749-6632.1994.tb44361.x. Ann N Y Acad Sci. 1994. PMID: 7832509 Review.
-
Recent Advances in Metabolic Engineering for the Biosynthesis of Phosphoenol Pyruvate-Oxaloacetate-Pyruvate-Derived Amino Acids.Molecules. 2024 Jun 18;29(12):2893. doi: 10.3390/molecules29122893. Molecules. 2024. PMID: 38930958 Free PMC article. Review.
Cited by
-
Antibacterial Effect and Mechanism of Chelerythrine on Xanthomonas oryzae pv. oryzae.Microorganisms. 2025 Apr 21;13(4):953. doi: 10.3390/microorganisms13040953. Microorganisms. 2025. PMID: 40284789 Free PMC article.
-
Innova 2020: A Follow-Up Study of the Fecal Microbiota of Infants Using a Novel Infant Formula between 6 Months and 12 Months of Age.Int J Mol Sci. 2023 Apr 17;24(8):7392. doi: 10.3390/ijms24087392. Int J Mol Sci. 2023. PMID: 37108555 Free PMC article.
-
Isolation and Characterization of High-Temperature-Tolerant Mutants of Bradyrhizobium diazoefficiens USDA110 by Carbon-Ion Beam Irradiation.Microorganisms. 2024 Sep 2;12(9):1819. doi: 10.3390/microorganisms12091819. Microorganisms. 2024. PMID: 39338493 Free PMC article.
-
Catabolism of 2-keto-3-deoxy-galactonate and the production of its enantiomers.Appl Microbiol Biotechnol. 2024 Jul 2;108(1):403. doi: 10.1007/s00253-024-13235-x. Appl Microbiol Biotechnol. 2024. PMID: 38954014 Free PMC article. Review.
-
Advances in the optimization of central carbon metabolism in metabolic engineering.Microb Cell Fact. 2023 Apr 21;22(1):76. doi: 10.1186/s12934-023-02090-6. Microb Cell Fact. 2023. PMID: 37085866 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

