Ensemble switching unveils a kinetic rheostat mechanism of the eukaryotic thiamine pyrophosphate riboswitch
- PMID: 33863818
- PMCID: PMC8208051
- DOI: 10.1261/rna.075937.120
Ensemble switching unveils a kinetic rheostat mechanism of the eukaryotic thiamine pyrophosphate riboswitch
Abstract
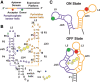
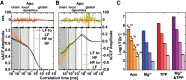
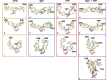
Thiamine pyrophosphate (TPP) riboswitches regulate thiamine metabolism by inhibiting the translation of enzymes essential to thiamine synthesis pathways upon binding to thiamine pyrophosphate in cells across all domains of life. Recent work on the Arabidopsis thaliana TPP riboswitch suggests a multistep TPP binding process involving multiple riboswitch configurational ensembles and Mg2+ dependence underlies the mechanism of TPP recognition and subsequent transition to the expression-inhibiting state of the aptamer domain followed by changes in the expression platform. However, details of the relationship between TPP riboswitch conformational changes and interactions with TPP and Mg2+ in the aptamer domain constituting this mechanism are unknown. Therefore, we integrated single-molecule multiparameter fluorescence and force spectroscopy with atomistic molecular dynamics simulations and found that conformational transitions within the aptamer domain's sensor helices associated with TPP and Mg2+ ligand binding occurred between at least five different ensembles on timescales ranging from µs to ms. These dynamics are orders of magnitude faster than the 10 sec-timescale folding kinetics associated with expression-state switching in the switch helix. Together, our results show that a TPP and Mg2+ dependent mechanism determines dynamic configurational state ensemble switching of the aptamer domain's sensor helices that regulate the switch helix's stability, which ultimately may lead to the expression-inhibiting state of the riboswitch. Additionally, we propose that two pathways exist for ligand recognition and that this mechanism underlies a kinetic rheostat-like behavior of the Arabidopsis thaliana TPP riboswitch.
Keywords: TPP riboswitch; discrete molecular dynamic simulations; fluorescence correlation spectroscopy; single molecule FRET.
© 2021 Ma et al.; Published by Cold Spring Harbor Laboratory Press for the RNA Society.
Figures






References
-
- Andersen HC. 1980. Molecular dynamics simulations at constant pressure and/or temperature. J Chem Phys 72: 2384–2393. 10.1063/1.439486 - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
