Interleukin-11-expressing fibroblasts have a unique gene signature correlated with poor prognosis of colorectal cancer
- PMID: 33863879
- PMCID: PMC8052408
- DOI: 10.1038/s41467-021-22450-3
Interleukin-11-expressing fibroblasts have a unique gene signature correlated with poor prognosis of colorectal cancer
Abstract
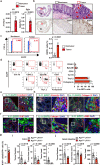
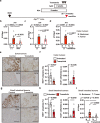
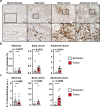
Interleukin (IL)-11 is a member of the IL-6 family of cytokines and is involved in multiple cellular responses, including tumor development. However, the origin and functions of IL-11-producing (IL-11+) cells are not fully understood. To characterize IL-11+ cells in vivo, we generate Il11 reporter mice. IL-11+ cells appear in the colon in murine tumor and acute colitis models. Il11ra1 or Il11 deletion attenuates the development of colitis-associated colorectal cancer. IL-11+ cells express fibroblast markers and genes associated with cell proliferation and tissue repair. IL-11 induces the activation of colonic fibroblasts and epithelial cells through phosphorylation of STAT3. Human cancer database analysis reveals that the expression of genes enriched in IL-11+ fibroblasts is elevated in human colorectal cancer and correlated with reduced recurrence-free survival. IL-11+ fibroblasts activate both tumor cells and fibroblasts via secretion of IL-11, thereby constituting a feed-forward loop between tumor cells and fibroblasts in the tumor microenvironment.
Conflict of interest statement
The authors declare no competing interests.
Figures







References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

