Evaluation of the C60 biodistribution in mice in a micellar ExtraOx form and in an oil solution
- PMID: 33863918
- PMCID: PMC8052328
- DOI: 10.1038/s41598-021-87014-3
Evaluation of the C60 biodistribution in mice in a micellar ExtraOx form and in an oil solution
Abstract
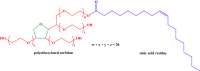
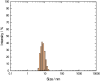
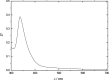
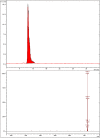
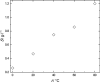
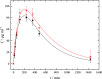
The article is devoted to the study of the pharmacokinetics of fullerene C60 in oil and micellar forms, analysis of its content in blood, liver, lungs, kidneys, heart, brain, adrenal glands, thymus, testicles, and spleen. The highest accumulation of C60 was found in the liver and adrenal glands. As a result of the studies carried out, it was shown that the bioavailability of C60 in the micellar form is higher than that in an oil solution.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Friedman SH, et al. Inhibition of the HIV-1 protease by fullerene derivatives: model building studies and experimental verification. J. Am. Chem. Soc. 1993;115:6506–6509. doi: 10.1021/ja00068a005. - DOI
-
- Piotrovsky LB, Kiselev OI. Fullerenes and viruses. Fullerenes Nanotub. Carbon Nanostruct. 2005;12:397–403. doi: 10.1081/FST-120027198. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

