Locally Advanced Pancreatic Cancer: Percutaneous Management Using Ablation, Brachytherapy, Intra-arterial Chemotherapy, and Intra-tumoral Immunotherapy
- PMID: 33864144
- PMCID: PMC8052234
- DOI: 10.1007/s11912-021-01057-3
Locally Advanced Pancreatic Cancer: Percutaneous Management Using Ablation, Brachytherapy, Intra-arterial Chemotherapy, and Intra-tumoral Immunotherapy
Abstract
Purpose of review: Pancreatic ductal adenocarcinoma (PDAC) is one of the most aggressive neoplasms, bearing a terrible prognosis. Stage III tumors, also known as locally advanced pancreatic cancer (LAPC), are unresectable, and current palliative chemotherapy regimens have only modestly improved survival in these patients. At this stage of disease, interventional techniques may be of value and further prolong life. The aim of this review was to explore current literature on locoregional percutaneous management for LAPC.
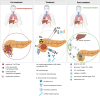
Recent findings: Locoregional percutaneous interventional techniques such as ablation, brachytherapy, and intra-arterial chemotherapy possess cytoreductive abilities and have the potential to increase survival. In addition, recent research demonstrates the immunomodulatory capacities of these treatments. This immune response may be leveraged by combining the interventional techniques with intra-tumoral immunotherapy, possibly creating a durable anti-tumor effect. This multimodality treatment approach is currently being examined in several ongoing clinical trials. The use of certain interventional techniques appears to improve survival in LAPC patients and may work synergistically when combined with immunotherapy. However, definitive conclusions can only be made when large prospective (randomized controlled) trials confirm these results.
Keywords: Ablation; Brachytherapy; Cryoablation; Intra-arterial chemotherapy; Intra-tumoral immunotherapy; Irreversible electroporation; Locally advanced pancreatic cancer; Microwave ablation; Radiofrequency ablation.
Conflict of interest statement
Florentine E.F. Timmer, Bart Geboers, Sanne Nieuwenhuizen, Evelien A.C. Schouten, Madelon Dijkstra, Jan J.J. de Vries, M. Petrousjka van den Tol, Tanja D. de Gruijl, and Hester J. Scheffer declare no conflict of interest. Martijn R. Meijerink is a paid consultant for AngioDynamics, and received NanoKnife needle electrode compensation for clinical trials from AngioDynamics.
Figures
Similar articles
-
Needle-guided ablation of locally advanced pancreatic cancer: cytoreduction or immunomodulation by in vivo vaccination?Chin Clin Oncol. 2019 Dec;8(6):61. doi: 10.21037/cco.2019.10.05. Epub 2019 Nov 25. Chin Clin Oncol. 2019. PMID: 31865711 Review.
-
Minimally invasive image-guided therapy of primary and metastatic pancreatic cancer.World J Gastroenterol. 2021 Jul 21;27(27):4322-4341. doi: 10.3748/wjg.v27.i27.4322. World J Gastroenterol. 2021. PMID: 34366607 Free PMC article. Review.
-
Value of CT-Guided Percutaneous Irreversible Electroporation Added to FOLFIRINOX Chemotherapy in Locally Advanced Pancreatic Cancer: A Post Hoc Comparison.J Vasc Interv Radiol. 2020 Oct;31(10):1600-1608. doi: 10.1016/j.jvir.2020.02.024. Epub 2020 Aug 27. J Vasc Interv Radiol. 2020. PMID: 32861569
-
Comparison of combination therapies in the management of locally advanced pancreatic cancer: Induction chemotherapy followed by irreversible electroporation vs radiofrequency ablation.Cancer Med. 2020 Jul;9(13):4699-4710. doi: 10.1002/cam4.3119. Epub 2020 May 15. Cancer Med. 2020. PMID: 32410380 Free PMC article.
-
Ablation treatments in unresectable pancreatic cancer.Minerva Chir. 2019 Jun;74(3):263-269. doi: 10.23736/S0026-4733.18.07881-1. Epub 2019 Jan 2. Minerva Chir. 2019. PMID: 30600963 Review.
Cited by
-
Rational Nanomedicine Design Enhances Clinically Physical Treatment-Inspired or Combined Immunotherapy.Adv Sci (Weinh). 2022 Oct;9(29):e2203921. doi: 10.1002/advs.202203921. Epub 2022 Aug 24. Adv Sci (Weinh). 2022. PMID: 36002305 Free PMC article. Review.
-
Interventional Radiology in the Treatment of Pancreatic Adenocarcinoma: Present and Future Perspectives.Life (Basel). 2023 Mar 20;13(3):835. doi: 10.3390/life13030835. Life (Basel). 2023. PMID: 36983990 Free PMC article. Review.
-
Interventional Oncology and Immuno-Oncology: Current Challenges and Future Trends.Int J Mol Sci. 2023 Apr 16;24(8):7344. doi: 10.3390/ijms24087344. Int J Mol Sci. 2023. PMID: 37108507 Free PMC article. Review.
-
Intratumoral Immunotherapy and Tumor Ablation: A Local Approach with Broad Potential.Cancers (Basel). 2022 Mar 30;14(7):1754. doi: 10.3390/cancers14071754. Cancers (Basel). 2022. PMID: 35406525 Free PMC article. Review.
-
Clinical practice guidelines for the interventional treatment of advanced pancreatic cancer (5th edition).J Interv Med. 2021 Aug 14;4(4):159-171. doi: 10.1016/j.jimed.2021.08.001. eCollection 2021 Nov. J Interv Med. 2021. PMID: 35586384 Free PMC article.
References
-
- Ducreux M, Cuhna AS, Caramella C, Hollebecque A, Burtin P, Goéré D, Seufferlein T, Haustermans K, van Laethem J, Conroy T, Arnold D, ESMO Guidelines Committee Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(Suppl 5):v56–v68. doi: 10.1093/annonc/mdv295. - DOI - PubMed
-
- •• Ruarus AH, Vroomen L, Geboers B, van Veldhuisen E, Puijk RS, Nieuwenhuizen S, et al. Percutaneous Irreversible Electroporation in Locally Advanced and Recurrent Pancreatic Cancer (PANFIRE-2): a multicenter, prospective, single-arm, phase II study. Radiology. 2019:191109 This is the largest prospective trial to date on percutaneous IRE for LAPC patients, thus holding valuable data on the potential of the technique. They demonstrated survival after diagnosis of 17 months and after treatment of 9.6 months. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

