DNA repair in primordial follicle oocytes following cisplatin treatment
- PMID: 33864208
- PMCID: PMC8266985
- DOI: 10.1007/s10815-021-02184-3
DNA repair in primordial follicle oocytes following cisplatin treatment
Abstract
Purpose: Genotoxic chemotherapy and radiotherapy can cause DNA double stranded breaks (DSBs) in primordial follicle (PMF) oocytes, which then undergo apoptosis. The development of effective new fertility preservation agents has been hampered, in part, by a limited understanding of DNA repair in PMF oocytes. This study investigated the induction of classical DSB repair pathways in the follicles of wild type (WT) and apoptosis-deficient Puma-/- mice in response to DSBs caused by the chemotherapy agent cisplatin.
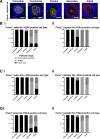
Methods: Adult C57BL/6 WT and Puma-/- mice were injected i.p. with saline or cisplatin (5 mg/kg); ovaries were harvested at 8 or 24 h. Follicles were counted, and H2A histone family member (γH2AX) immunofluorescence used to demonstrate DSBs. DNA repair protein RAD51 homolog 1 (RAD51) and DNA-dependent protein kinase, catalytic subunit (DNA-PKcs) immunofluorescence were used to identify DNA repair pathways utilised.
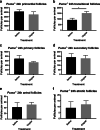
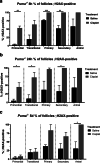
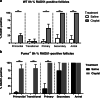
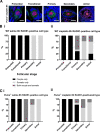
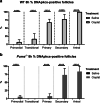
Results: Puma-/- mice retained 100% of follicles 24 h after cisplatin treatment. Eight hours post-treatment, γH2AX immunofluorescence showed DSBs across follicular stages in Puma-/- mice; staining returned to control levels in PMFs within 5 days, suggesting repair of PMF oocytes in this window. RAD51 immunofluorescence eight hours post-cisplatin was positive in damaged cell types in both WT and Puma-/- mice, demonstrating induction of the homologous recombination pathway. In contrast, DNA-PKcs staining were rarely observed in PMFs, indicating non-homologous end joining plays an insignificant role.
Conclusion: PMF oocytes are able to conduct high-fidelity repair of DNA damage accumulated during chemotherapy. Therefore, apoptosis inhibition presents a viable strategy for fertility preservation in women undergoing treatment.
Keywords: Apoptosis; Chemotherapy; DNA repair; Fertility; Follicle; Oocyte.
Conflict of interest statement
None to declare.
Figures
Similar articles
-
Cisplatin- and cyclophosphamide-induced primordial follicle depletion is caused by direct damage to oocytes.Mol Hum Reprod. 2019 Aug 1;25(8):433-444. doi: 10.1093/molehr/gaz020. Mol Hum Reprod. 2019. PMID: 30953068
-
Loss of PUMA protects the ovarian reserve during DNA-damaging chemotherapy and preserves fertility.Cell Death Dis. 2018 May 23;9(6):618. doi: 10.1038/s41419-018-0633-7. Cell Death Dis. 2018. PMID: 29795269 Free PMC article.
-
Xrcc5/KU80 is not required for the survival or activation of prophase-arrested oocytes in primordial follicles.Front Endocrinol (Lausanne). 2023 Oct 10;14:1268009. doi: 10.3389/fendo.2023.1268009. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37900135 Free PMC article.
-
BRCA-related ATM-mediated DNA double-strand break repair and ovarian aging.Hum Reprod Update. 2020 Jan 1;26(1):43-57. doi: 10.1093/humupd/dmz043. Hum Reprod Update. 2020. PMID: 31822904 Free PMC article. Review.
-
Mechanisms of DNA double strand break repair and chromosome aberration formation.Cytogenet Genome Res. 2004;104(1-4):14-20. doi: 10.1159/000077461. Cytogenet Genome Res. 2004. PMID: 15162010 Review.
Cited by
-
Endometrial stem cells alleviate cisplatin-induced ferroptosis of granulosa cells by regulating Nrf2 expression.Reprod Biol Endocrinol. 2024 Apr 11;22(1):41. doi: 10.1186/s12958-024-01208-8. Reprod Biol Endocrinol. 2024. PMID: 38605340 Free PMC article.
-
Reduced reproductive potential in young healthy women with hereditary breast and/or ovarian cancer syndrome.Commun Med (Lond). 2025 Mar 8;5(1):70. doi: 10.1038/s43856-025-00788-9. Commun Med (Lond). 2025. PMID: 40057639 Free PMC article.
-
TAp63 determines the fate of oocytes against DNA damage.Sci Adv. 2022 Dec 21;8(51):eade1846. doi: 10.1126/sciadv.ade1846. Epub 2022 Dec 21. Sci Adv. 2022. PMID: 36542718 Free PMC article.
-
BRCA Mutations and Fertility Preservation.Int J Mol Sci. 2023 Dec 22;25(1):204. doi: 10.3390/ijms25010204. Int J Mol Sci. 2023. PMID: 38203374 Free PMC article. Review.
-
The future of fertility preservation for women treated with chemotherapy.Reprod Fertil. 2023 Apr 1;4(2):e220123. doi: 10.1530/RAF-22-0123. Online ahead of print. Reprod Fertil. 2023. PMID: 37068157 Free PMC article.
References
-
- Byrne J, Fears TR, Gail MH, Pee D, Connelly RR, Austin DF, Holmes GF, Holmes FF, Latourette HB, Meigs JW, Strong LC, Myers MH, Mulvihill JJ. Early menopause in long-term survivors of cancer during adolescence. American journal of obstetrics and gynecology. 1992;166(3):788–793. doi: 10.1016/0002-9378(92)91335-8. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

