Effects of Gocovri (Amantadine) Extended Release Capsules on Non-Motor Symptoms in Patients with Parkinson's Disease and Dyskinesia
- PMID: 33864229
- PMCID: PMC8140024
- DOI: 10.1007/s40120-021-00246-3
Effects of Gocovri (Amantadine) Extended Release Capsules on Non-Motor Symptoms in Patients with Parkinson's Disease and Dyskinesia
Abstract
Introduction: Gocovri (amantadine) extended release capsules are approved for treatment of dyskinesia and as a levodopa adjunct for OFF episodes in patients with Parkinson's disease (PD). We report treatment-related effects on non-motor symptoms (NMS) assessed as secondary outcomes in two trials using the Movement Disorder Society-Unified Parkinson's Disease Rating Scale (MDS-UPDRS) Part I.
Methods: EASE LID and EASE LID 3 enrolled levodopa-treated patients with PD and ≥ 1 h/day ON time with troublesome dyskinesia. Patients were randomized to Gocovri (274 mg) or placebo taken daily at bedtime. Treatment differences from baseline to week 12 in MDS-UPDRS Part I were evaluated for the pooled population (N = 196) from both trials. Correlation analyses of NMS (MDS-UPDRS Part I) with dyskinesia using Unified Dyskinesia Rating Scale (UDysRS) scores were performed.
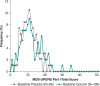
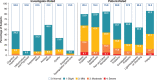
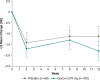
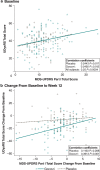
Results: For changes in the MDS-UPDRS Part I items, the treatment difference favored Gocovri in daytime sleepiness (P = 0.006) and depression (P = 0.049) scores, but favored placebo in cognitive impairment (P = 0.038), and hallucinations and psychosis (P < 0.001) scores. The treatment difference for the changes in total Part I score was -0.8, favoring Gocovri (P = 0.22). At baseline, MDS-UPDRS Part I modestly correlated with UDysRS score (r +0.25, P < 0.001), and improvement in NMS correlated with improvement in dyskinesia at week 12 for Gocovri (r +0.39, P < 0.001) but not placebo (r +0.12, P = 0.29). The most commonly reported adverse events for Gocovri were hallucination (21%); dizziness, dry mouth, and peripheral edema (16% each); and constipation, falls, and orthostatic hypotension (13% each).
Conclusion: This post hoc analysis shows potential benefit with Gocovri treatment for the NMS of daytime sleepiness and depression in dyskinetic PD patients. Overall, improvement in NMS scores correlated with improvement in dyskinesia.
Trial registration: ClinicalTrials.gov identifiers: NCT02136914 and NCT02274766.
Keywords: Amantadine; Depression; Depressive disorder; Dyskinesias; Hallucinations; Parkinson’s disease; Sleep.
Figures

Similar articles
-
Effects of Gocovri (Amantadine) Extended-Release Capsules on Motor Aspects of Experiences of Daily Living in People with Parkinson's Disease and Dyskinesia.Neurol Ther. 2021 Dec;10(2):739-751. doi: 10.1007/s40120-021-00256-1. Epub 2021 May 22. Neurol Ther. 2021. PMID: 34024025 Free PMC article.
-
EASE LID 2: A 2-Year Open-Label Trial of Gocovri (Amantadine) Extended Release for Dyskinesia in Parkinson's Disease.J Parkinsons Dis. 2020;10(2):543-558. doi: 10.3233/JPD-191841. J Parkinsons Dis. 2020. PMID: 31929122 Free PMC article. Clinical Trial.
-
Pooled Analyses of Phase III Studies of ADS-5102 (Amantadine) Extended-Release Capsules for Dyskinesia in Parkinson's Disease.CNS Drugs. 2018 Apr;32(4):387-398. doi: 10.1007/s40263-018-0498-4. CNS Drugs. 2018. PMID: 29532440 Free PMC article. Clinical Trial.
-
Amantadine Extended-Release (GOCOVRI™): A Review in Levodopa-Induced Dyskinesia in Parkinson's Disease.CNS Drugs. 2018 Aug;32(8):797-806. doi: 10.1007/s40263-018-0552-2. CNS Drugs. 2018. PMID: 30088203 Review.
-
Potential utility of amantadine DR/ER in persons with Parkinson's disease meeting 5-2-1 criteria for device aided therapy.Clin Park Relat Disord. 2021 Dec 8;6:100123. doi: 10.1016/j.prdoa.2021.100123. eCollection 2022. Clin Park Relat Disord. 2021. PMID: 35059622 Free PMC article. Review.
Cited by
-
Moving to a non-dopaminergic approach for the treatment of OFF fluctuations in Parkinson's disease.Clin Park Relat Disord. 2025 Jan 27;12:100303. doi: 10.1016/j.prdoa.2025.100303. eCollection 2025. Clin Park Relat Disord. 2025. PMID: 39968317 Free PMC article. Review.
-
Insomnia in Parkinson's Disease: Causes, Consequences, and Therapeutic Approaches.Mol Neurobiol. 2025 Feb;62(2):2292-2313. doi: 10.1007/s12035-024-04400-4. Epub 2024 Aug 5. Mol Neurobiol. 2025. PMID: 39103716 Free PMC article. Review.
-
Glutamate Receptors and C-ABL Inhibitors: A New Therapeutic Approach for Parkinson's Disease.Cent Nerv Syst Agents Med Chem. 2024;24(1):22-44. doi: 10.2174/0118715249268627231206115942. Cent Nerv Syst Agents Med Chem. 2024. PMID: 38273763 Review.
-
Excessive Daytime Sleepiness in Parkinson's Disease.Nat Sci Sleep. 2022 Sep 7;14:1589-1609. doi: 10.2147/NSS.S375098. eCollection 2022. Nat Sci Sleep. 2022. PMID: 36105924 Free PMC article. Review.
-
An Update on Nondopaminergic Treatments for Motor and Non-motor Symptoms of Parkinson's Disease.Curr Neuropharmacol. 2023;21(8):1806-1826. doi: 10.2174/1570159X20666220222150811. Curr Neuropharmacol. 2023. PMID: 35193486 Free PMC article. Review.
References
-
- Chaudhuri KR, Martinez-Martin P, Schapira AH, Stocchi F, Sethi K, Odin P, et al. International multicenter pilot study of the first comprehensive self-completed nonmotor symptoms questionnaire for Parkinson's disease: the NMSQuest study. Mov Disord. 2006;21(7):916–923. doi: 10.1002/mds.20844. - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

