Upregulation of eIF4E, but not other translation initiation factors, in dendritic spines during memory formation
- PMID: 33864263
- PMCID: PMC8165027
- DOI: 10.1002/cne.25158
Upregulation of eIF4E, but not other translation initiation factors, in dendritic spines during memory formation
Abstract
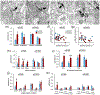
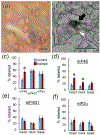
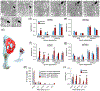
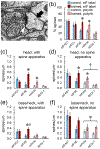
Local translation can provide a rapid, spatially targeted supply of new proteins in distal dendrites to support synaptic changes that underlie learning. Learning and memory are especially sensitive to manipulations of translational control mechanisms, particularly those that target the initiation step, and translation initiation at synapses could be a means of maintaining synapse specificity during plasticity. Initiation predominantly occurs via recruitment of ribosomes to the 5' mRNA cap by complexes of eukaryotic initiation factors (eIFs), and the interaction between eIF4E and eIF4G1 is a particularly important target of translational control pathways. Pharmacological inhibition of eIF4E-eIF4G1 binding impairs formation of memory for aversive Pavlovian conditioning as well as the accompanying increase in polyribosomes in the heads of dendritic spines in the lateral amygdala (LA). This is consistent with a role for initiation at synapses in memory formation, but whether eIFs are even present near synapses is unknown. To determine whether dendritic spines contain eIFs and whether eIF distribution is affected by learning, we combined immunolabeling with serial section transmission electron microscopy (ssTEM) volume reconstructions of LA dendrites after Pavlovian conditioning. Labeling for eIF4E, eIF4G1, and eIF2α-another key target of regulation-occurred in roughly half of dendritic spines, but learning effects were only found for eIF4E, which was upregulated in the heads of dendritic spines. Our results support the possibility of regulated translation initiation as a means of synapse-specific protein targeting during learning and are consistent with the model of eIF4E availability as a central point of control.
Keywords: amygdala; dendritic spine; electron microscopy; eukaryotic initiation factor; fear conditioning; learning and memory; polyribosome.
© 2021 Wiley Periodicals LLC.
Conflict of interest statement
Figures



Similar articles
-
Accumulation of Polyribosomes in Dendritic Spine Heads, But Not Bases and Necks, during Memory Consolidation Depends on Cap-Dependent Translation Initiation.J Neurosci. 2017 Feb 15;37(7):1862-1872. doi: 10.1523/JNEUROSCI.3301-16.2017. Epub 2017 Jan 13. J Neurosci. 2017. PMID: 28087764 Free PMC article.
-
Shifting patterns of polyribosome accumulation at synapses over the course of hippocampal long-term potentiation.Hippocampus. 2018 Jun;28(6):416-430. doi: 10.1002/hipo.22841. Epub 2018 Apr 16. Hippocampus. 2018. PMID: 29575288 Free PMC article.
-
Synapses lacking astrocyte appear in the amygdala during consolidation of Pavlovian threat conditioning.J Comp Neurol. 2014 Jun 15;522(9):2152-63. doi: 10.1002/cne.23523. J Comp Neurol. 2014. PMID: 24338694 Free PMC article.
-
Structure and functions of the translation initiation factor eIF4E and its role in cancer development and treatment.J Genet Genomics. 2018 Jan 20;45(1):13-24. doi: 10.1016/j.jgg.2018.01.003. Epub 2018 Feb 1. J Genet Genomics. 2018. PMID: 29396141 Review.
-
The emerging roles of translation factor eIF4E in the nucleus.Differentiation. 2002 Mar;70(1):10-22. doi: 10.1046/j.1432-0436.2002.700102.x. Differentiation. 2002. PMID: 11963652 Review.
Cited by
-
ZIP9 mediates the effects of DHT on learning, memory and hippocampal synaptic plasticity of male Tfm and APP/PS1 mice.Front Endocrinol (Lausanne). 2023 May 25;14:1139874. doi: 10.3389/fendo.2023.1139874. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37305050 Free PMC article.
-
Behavioral screening reveals a conserved residue in Y-Box RNA-binding protein required for associative learning and memory in C. elegans.PLoS Genet. 2024 Oct 18;20(10):e1011443. doi: 10.1371/journal.pgen.1011443. eCollection 2024 Oct. PLoS Genet. 2024. PMID: 39423228 Free PMC article.
-
Rehydration of Freeze Substituted Brain Tissue for Pre-embedding Immunoelectron Microscopy.Microsc Microanal. 2023 Sep 29;29(5):1694-1704. doi: 10.1093/micmic/ozad077. Microsc Microanal. 2023. PMID: 37584524 Free PMC article.
-
Behavioral screening of conserved RNA-binding proteins reveals CEY-1/YBX RNA-binding protein dysfunction leads to impairments in memory and cognition.bioRxiv [Preprint]. 2024 May 2:2024.01.05.574402. doi: 10.1101/2024.01.05.574402. bioRxiv. 2024. PMID: 38260399 Free PMC article. Preprint.
-
Persistent up-regulation of polyribosomes at synapses during long-term memory, reconsolidation, and extinction of associative memory.Learn Mem. 2022 Jul 26;29(8):192-202. doi: 10.1101/lm.053577.122. Print 2022 Aug. Learn Mem. 2022. PMID: 35882501 Free PMC article.
References
-
- Asaki C, Usuda N, Nakazawa A, Kametani K, Suzuki T (2003) Localization of translational components at the ultramicroscopic level at postsynaptic sites of the rat brain. Brain Res 972:168–176. - PubMed
-
- Biever A, Glock C, Tushev G, Ciirdaeva E, Dalmay T, Langer JD, Schuman EM (2020) Monosomes actively translate synaptic mRNAs in neuronal processes. Science 367:eaay4991. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- MH094965/MH/NIMH NIH HHS/United States
- MH119517/MH/NIMH NIH HHS/United States
- R21 MH094965/MH/NIMH NIH HHS/United States
- R01 NS047384/NS/NINDS NIH HHS/United States
- NS034007/NS/NINDS NIH HHS/United States
- R37 NS034007/NS/NINDS NIH HHS/United States
- NS047384/NS/NINDS NIH HHS/United States
- R29 NS034007/NS/NINDS NIH HHS/United States
- R01 NS034007/NS/NINDS NIH HHS/United States
- R21 MH119517/MH/NIMH NIH HHS/United States
- R01 NS086933/NS/NINDS NIH HHS/United States
- MH083583/MH/NIMH NIH HHS/United States
- F32 MH083583/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

