Cocaine experience induces functional adaptations in astrocytes: Implications for synaptic plasticity in the nucleus accumbens shell
- PMID: 33864336
- PMCID: PMC9446435
- DOI: 10.1111/adb.13042
Cocaine experience induces functional adaptations in astrocytes: Implications for synaptic plasticity in the nucleus accumbens shell
Abstract
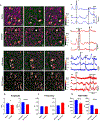
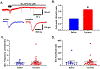
Astrocytes have become established as an important regulator of neuronal activity in the brain. Accumulating literature demonstrates that cocaine self-administration in rodent models induces structural changes within astrocytes that may influence their interaction with the surrounding neurons. Here, we provide evidence that cocaine impacts astrocytes at the functional level and alters neuronal sensitivity to astrocyte-derived glutamate. We report that a 14-day period of short access to cocaine (2 h/day) decreases spontaneous astrocytic Ca2+ transients and precipitates changes in astrocyte network activity in the nucleus accumbens shell. This is accompanied by increased prevalence of slow inward currents, a physiological marker of neuronal activation by astrocytic glutamate, in a subset of medium spiny neurons. Within, but not outside, of this subset, we observe an increase in duration and frequency of N-methyl-D-aspartate (NMDA) receptor-mediated synaptic events. Additionally, we find that the link between synaptic NMDA receptor plasticity and neuron sensitivity to astrocytic glutamate is maintained independent of drug exposure and is observed in both cocaine and saline control animals. Imaging analyses of neuronal Ca2+ activity show no effect of cocaine self-administration on individual cells or on neuronal network activity in brain slices. Therefore, our data indicate that cocaine self-administration promotes astrocyte-specific functional changes that can be linked to increased glutamate-mediated coupling with principal neurons in the nucleus accumbens. Such coupling may be spatially restricted as it does not result in a broad impact on network structure of local neuronal circuits.
Keywords: astrocyte; cocaine self-administration; synaptic plasticity.
© 2021 Society for the Study of Addiction.
Figures





References
-
- Belanger M, Allaman I, Magistretti PJ. Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab. 2011;14(6):724–738. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

