Aβ1-16 controls synaptic vesicle pools at excitatory synapses via cholinergic modulation of synapsin phosphorylation
- PMID: 33864480
- PMCID: PMC8233295
- DOI: 10.1007/s00018-021-03835-5
Aβ1-16 controls synaptic vesicle pools at excitatory synapses via cholinergic modulation of synapsin phosphorylation
Abstract
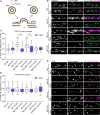
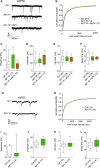
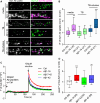
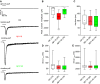
Amyloid beta (Aβ) is linked to the pathology of Alzheimer's disease (AD). At physiological concentrations, Aβ was proposed to enhance neuroplasticity and memory formation by increasing the neurotransmitter release from presynapse. However, the exact mechanisms underlying this presynaptic effect as well as specific contribution of endogenously occurring Aβ isoforms remain unclear. Here, we demonstrate that Aβ1-42 and Aβ1-16, but not Aβ17-42, increased size of the recycling pool of synaptic vesicles (SV). This presynaptic effect was driven by enhancement of endogenous cholinergic signalling via α7 nicotinic acetylcholine receptors, which led to activation of calcineurin, dephosphorylation of synapsin 1 and consequently resulted in reorganization of functional pools of SV increasing their availability for sustained neurotransmission. Our results identify synapsin 1 as a molecular target of Aβ and reveal an effect of physiological concentrations of Aβ on cholinergic modulation of glutamatergic neurotransmission. These findings provide new mechanistic insights in cholinergic dysfunction observed in AD.
Keywords: Alpha7 nicotinic acetylcholine receptor; Amyloid beta; Synapsin 1; Synaptic vesicle dynamics.
Conflict of interest statement
The authors have no competitive interests to declare.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

