Safety, tolerability, and immunogenicity of the respiratory syncytial virus prefusion F subunit vaccine DS-Cav1: a phase 1, randomised, open-label, dose-escalation clinical trial
- PMID: 33864736
- PMCID: PMC8487912
- DOI: 10.1016/S2213-2600(21)00098-9
Safety, tolerability, and immunogenicity of the respiratory syncytial virus prefusion F subunit vaccine DS-Cav1: a phase 1, randomised, open-label, dose-escalation clinical trial
Abstract
Background: Multiple active vaccination approaches have proven ineffective in reducing the substantial morbidity and mortality caused by respiratory syncytial virus (RSV) in infants and older adults (aged ≥65 years). A vaccine conferring a substantial and sustainable boost in neutralising activity is required to protect against severe RSV disease. To that end, we evaluated the safety and immunogenicity of DS-Cav1, a prefusion F subunit vaccine.
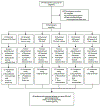
Methods: In this randomised, open-label, phase 1 clinical trial, the stabilised prefusion F vaccine DS-Cav1 was evaluated for dose, safety, tolerability, and immunogenicity in healthy adults aged 18-50 years at a single US site. Participants were assigned to receive escalating doses of either 50 μg, 150 μg, or 500 μg DS-Cav1 at weeks 0 and 12, and were randomly allocated in a 1:1 ratio within each dose group to receive the vaccine with or without aluminium hydroxide (AlOH) adjuvant. After 71 participants had been randomised, the protocol was amended to allow some participants to receive a single vaccination at week 0. The primary objectives evaluated the safety and tolerability at every dose within 28 days following each injection. Neutralising activity and RSV F-binding antibodies were evaluated from week 0 to week 44 as secondary and exploratory objectives. Safety was assessed in all participants who received at least one vaccine dose; secondary and exploratory immunogenicity analysis included all participants with available data at a given visit. The trial is registered with ClinicalTrials.gov, NCT03049488, and is complete and no longer recruiting.
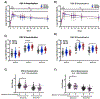
Findings: Between Feb 21, 2017, and Nov 29, 2018, 244 participants were screened for eligibility and 95 were enrolled to receive DS-Cav1 at the 50 μg (n=30, of which n=15 with AlOH), 150 μg (n=35, of which n=15 with AlOH), or 500 μg (n=30, of which n=15 with AlOH) doses. DS-Cav1 was safe and well tolerated and no serious vaccine-associated adverse events deemed related to the vaccine were identified. DS-Cav1 vaccination elicited robust neutralising activity and binding antibodies by 4 weeks after a single vaccination (p<0·0001 for F-binding and neutralising antibodies). In analyses of exploratory endpoints at week 44, pre-F-binding IgG and neutralising activity were significantly increased compared with baseline in all groups. At week 44, RSV A neutralising activity was 3·1 fold above baseline in the 50 μg group, 3·8 fold in the 150 μg group, and 4·5 fold in the 500 μg group (p<0·0001). RSV B neutralising activity was 2·8 fold above baseline in the 50 μg group, 3·4 fold in the 150 μg group, and 3·7 fold in the 500 μg group (p<0·0001). Pre-F-binding IgG remained significantly 3·2 fold above baseline in the 50 μg group, 3·4 fold in the 150 μg group, and 4·0 fold in the 500 μg group (p<0·0001). Pre-F-binding serum IgA remained 4·1 fold above baseline in the 50 μg group, 4·3 fold in the 150 μg group, and 4·8 fold in the 500 μg group (p<0·0001). Although a higher vaccine dose or second immunisation elicited a transient advantage compared with lower doses or a single immunisation, neither significantly impacted long-term neutralisation. There was no long-term effect of dose, number of vaccinations, or adjuvant on neutralising activity.
Interpretation: In this phase 1 study, DS-Cav1 vaccination was safe and well tolerated. DS-Cav1 vaccination elicited a robust boost in RSV F-specific antibodies and neutralising activity that was sustained above baseline for at least 44 weeks. A single low-dose of pre-F immunisation of antigen-experienced individuals might confer protection that extends throughout an entire RSV season.
Funding: The National Institutes of Allergy and Infectious Diseases.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests MC and BSG are inventors on patents for the stabilisation of the RSV F protein. The other authors declared no competing interests.
Figures

References
-
- Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med 2005; 352(17): 1749–59. - PubMed
-
- Shi T, Denouel A, Tietjen AK, et al.Global Disease Burden Estimates of Respiratory Syncytial Virus-Associated Acute Respiratory Infection in Older Adults in 2015: A Systematic Review and Meta-Analysis. J Infect Dis 2019. - PubMed
-
- Kim HW, Canchola JG, Brandt CD, et al.Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol 1969; 89(4): 422–34. - PubMed
-
- Chin J, Magoffin RL, Shearer LA, Schieble JH, Lennette EH. Field evaluation of a respiratory syncytial virus vaccine and a trivalent parainfluenza virus vaccine in a pediatric population. Am J Epidemiol 1969; 89(4): 449–63. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

