Abnormal development of cerebral arteries and veins in offspring of experimentally preeclamptic rats: Potential role in perinatal stroke
- PMID: 33864898
- PMCID: PMC8154742
- DOI: 10.1016/j.mad.2021.111491
Abnormal development of cerebral arteries and veins in offspring of experimentally preeclamptic rats: Potential role in perinatal stroke
Abstract
Preeclampsia, a hypertensive disorder of pregnancy, complicates up to 10 % of all pregnancies and increases the risk for perinatal stroke in offspring. The mechanism of this increase is unknown, but may involve vascular dysfunction. The goal of this study was to evaluate the effect of experimental preeclampsia (ePE) on cerebrovascular function in offspring to eludciate a possible mechanism for this association. Dams were fed a high cholesterol diet beginning on day 7 of gestation to induce experimental preeclampsia. Middle cerebral arteries (MCA) and the Vein of Galen (VoG) were isolated from pups from ePE dams and compared to pups from normal pregnant (NP) dams at postnatal days 16, 23, and 30 and studied pressurized in an arteriograph chamber. Markers of inflammation and oxidative stress were measured in serum. Our results suggest altered structure and function in both MCA and VoG of ePE pups. We also found evidence of systemic inflammation and oxidative stress in ePE pups. These findings provide a potential link between preeclampsia and the occurrence or severity of perinatal stroke.
Keywords: Cerebral vasculature; Middle cerebral artery; Offspring; Preeclampsia; Vein of galen.
Copyright © 2021 Elsevier B.V. All rights reserved.
Figures
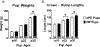
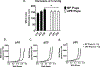
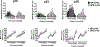
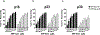
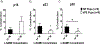
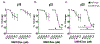
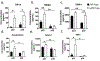
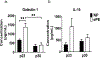
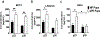
Similar articles
-
Differential Effects of LOX-1 Inhibition on Aortic Structure and Posterior Cerebral Artery Structure and Function in an Experimental Model of Preeclampsia.Reprod Sci. 2024 Oct;31(10):3004-3015. doi: 10.1007/s43032-024-01607-7. Epub 2024 Jun 10. Reprod Sci. 2024. PMID: 38858329
-
Impaired function of cerebral parenchymal arterioles in experimental preeclampsia.Microvasc Res. 2018 Sep;119:64-72. doi: 10.1016/j.mvr.2018.04.007. Epub 2018 Apr 26. Microvasc Res. 2018. PMID: 29705580 Free PMC article.
-
Predictive value of middle cerebral artery to uterine artery pulsatility index ratio in preeclampsia.Arch Gynecol Obstet. 2011 Aug;284(2):307-11. doi: 10.1007/s00404-010-1660-5. Epub 2010 Sep 2. Arch Gynecol Obstet. 2011. PMID: 20811899 Clinical Trial.
-
Cerebrovascular Dysfunction in Preeclamptic Pregnancies.Curr Hypertens Rep. 2015 Aug;17(8):64. doi: 10.1007/s11906-015-0575-8. Curr Hypertens Rep. 2015. PMID: 26126779 Free PMC article. Review.
-
Preeclampsia and health risks later in life: an immunological link.Semin Immunopathol. 2016 Nov;38(6):699-708. doi: 10.1007/s00281-016-0579-8. Epub 2016 Jun 23. Semin Immunopathol. 2016. PMID: 27339196 Review.
Cited by
-
A Changepoint Detection-Based General Methodology for Robust Signal Processing: An Application to Understand Preeclampsia's Mechanisms.Bioengineering (Basel). 2025 Jun 19;12(6):675. doi: 10.3390/bioengineering12060675. Bioengineering (Basel). 2025. PMID: 40564491 Free PMC article.
-
Taprenepag restores maternal-fetal interface homeostasis for the treatment of neurodevelopmental disorders.J Neuroinflammation. 2024 Nov 28;21(1):307. doi: 10.1186/s12974-024-03300-7. J Neuroinflammation. 2024. PMID: 39609821 Free PMC article.
-
Language Impairment in Children of Mothers with Gestational Diabetes, Preeclampsia, and Preterm Delivery: Current Hypothesis and Potential Underlying Mechanisms : Language Impartment and Pregnancy Complications.Adv Exp Med Biol. 2023;1428:245-267. doi: 10.1007/978-3-031-32554-0_11. Adv Exp Med Biol. 2023. PMID: 37466777
-
The Ever-Evolving Concept of the Neurovascular Unit.Stroke. 2023 Aug;54(8):2178-2180. doi: 10.1161/STROKEAHA.123.042705. Epub 2023 Jul 3. Stroke. 2023. PMID: 37395105 Free PMC article. No abstract available.
-
Abnormal cerebral microvascular perfusion and reactivity in female offspring of reduced uterine perfusion pressure (RUPP) mice model.J Cereb Blood Flow Metab. 2022 Dec;42(12):2318-2332. doi: 10.1177/0271678X221121872. Epub 2022 Aug 25. J Cereb Blood Flow Metab. 2022. PMID: 36008921 Free PMC article.
References
-
- Duley L. The global impact of pre-eclampsia and eclampsia. Semin Perinatol 2009;33:130–7. - PubMed
-
- Wallis AB, Saftlas AF, Hsia J, Atrash HK. Secular trends in the rates of preeclampsia, eclampsia, and gestational hypertension, United States, 1987–2004. Am J Hypertens 2008;21:521–6. - PubMed
-
- Hutcheon JA, Lisonkova S, Joseph KS. Epidemiology of pre-eclampsia and the other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol 2011;25:391–403. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

