Positive impact of oral hydroxychloroquine and povidone-iodine throat spray for COVID-19 prophylaxis: An open-label randomized trial
- PMID: 33864917
- PMCID: PMC8056783
- DOI: 10.1016/j.ijid.2021.04.035
Positive impact of oral hydroxychloroquine and povidone-iodine throat spray for COVID-19 prophylaxis: An open-label randomized trial
Abstract
Background: We examined whether existing licensed pharmacotherapies could reduce the spread of coronavirus disease 2019 (COVID-19).
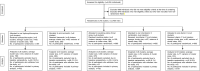
Methods: An open-label parallel randomized controlled trial was performed among healthy migrant workers quarantined in a large multi-storey dormitory in Singapore. Forty clusters (each defined as individual floors of the dormitory) were randomly assigned to receive a 42-day prophylaxis regimen of either oral hydroxychloroquine (400 mg once, followed by 200 mg/day), oral ivermectin (12 mg once), povidone-iodine throat spray (3 times/day, 270 μg/day), oral zinc (80 mg/day)/vitamin C (500 mg/day) combination, or oral vitamin C, 500 mg/day. The primary outcome was laboratory evidence of SARS-CoV-2 infection as shown by either: (1) a positive serologic test for SARS-CoV-2 antibody on day 42, or (2) a positive PCR test for SARS-CoV-2 at any time between baseline and day 42.
Results: A total of 3037 asymptomatic participants (mean age, 33.0 years; all men) who were seronegative to SARS-CoV-2 at baseline were included in the primary analysis. Follow-up was nearly complete (99.6%). Compared with vitamin C, significant absolute risk reductions (%, 98.75% confidence interval) were observed for oral hydroxychloroquine (21%, 2-42%) and povidone-iodine throat spray (24%, 7-39%). No statistically significant differences were observed with oral zinc/vitamin C combination (23%, -5 to +41%) and ivermectin (5%, -10 to +22%). Interruptions due to side effects were highest among participants who received zinc/vitamin C combination (6.9%), followed by vitamin C (4.7%), povidone-iodine (2.0%), and hydroxychloroquine (0.7%).
Conclusions: Chemoprophylaxis with either oral hydroxychloroquine or povidone-iodine throat spray was superior to oral vitamin C in reducing SARS-CoV-2 infection in young and healthy men.
Keywords: Hydroxychloroquine; Ivermectin; Povidone-iodine; SARS-CoV-2; Vitamin C; Zinc.
Copyright © 2021 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Figures
References
-
- Chen J.I., Yap J.C., Hsu L.Y., Teo Y.Y. COVID-19 and Singapore: from early response to circuit breaker. Ann Acad Med Singap. 2020;49:561–572. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

