LRRc17 controls BMSC senescence via mitophagy and inhibits the therapeutic effect of BMSCs on ovariectomy-induced bone loss
- PMID: 33865167
- PMCID: PMC8066428
- DOI: 10.1016/j.redox.2021.101963
LRRc17 controls BMSC senescence via mitophagy and inhibits the therapeutic effect of BMSCs on ovariectomy-induced bone loss
Abstract
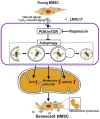
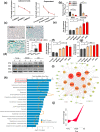
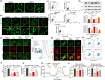
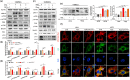
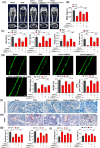
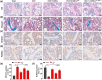
Senescence of bone marrow-derived mesenchymal stem cells (BMSCs) has been widely reported to be closely correlated with aging-related diseases, including osteoporosis (OP). Moreover, the beneficial functions of BMSCs decline with age, limiting their therapeutic efficacy in OP. In the present study, using RNA sequencing (RNA-Seq), we found that leucine-rich repeat containing 17 (LRRc17) expression in BMSCs was highly positively correlated with age. Therefore, we investigated whether LRRc17 knockdown could rejuvenate aged MSCs and increase their therapeutic efficacy in OP. Consistent with the RNA-Seq results, the protein expression of LRRc17 in senescent BMSCs was significantly increased, whereas LRRc17 knockdown inhibited cell apoptosis and reduced the expression of age-related proteins and G2 and S phase quiescence. Furthermore, LRRc17 knockdown shifted BMSCs from adipogenic to osteogenic differentiation, indicating the critical role of LRRc17 in BMSC senescence and differentiation. Additionally, similar to rapamycin (RAPA) treatment, LRRc17 knockdown activated mitophagy via inhibition of the mTOR/PI3K pathway, which consequently reduced mitochondrial dysfunction and inhibited BMSC senescence. However, the effects of LRRc17 knockdown were significantly blocked by the autophagy inhibitor hydroxychloroquine (HCQ), demonstrating that LRRc17 knockdown prevented BMSC senescence by activating mitophagy. In vivo, compared with untransfected aged mouse-derived BMSCs (O-BMSCs), O-BMSCs transfected with sh-LRRc17 showed effective amelioration of ovariectomy (OVX)-induced bone loss. Collectively, these results indicated that LRRc17 knockdown rejuvenated senescent BMSCs and thus enhanced their therapeutic efficacy in OP by activating autophagy.
Keywords: Aging; BMSCs; LRRc17; Mitophagy; Osteoporosis.
Copyright © 2021 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare no potential conflicts of interest.
Figures


References
-
- Li Y., Wu Q., Wang Y., Li L., Bu H., Bao J. Senescence of mesenchymal stem cells (Review) Int. J. Mol. Med. 2017;39:775–782. - PubMed
-
- Ren X., Hu B., Song M., Ding Z., Dang Y., Liu Z. Maintenance of nucleolar homeostasis by CBX4 alleviates senescence and osteoarthritis. Cell Rep. 2019;26:3643–3656 e3647. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

