Prediction of survival in amyotrophic lateral sclerosis: a nationwide, Danish cohort study
- PMID: 33865343
- PMCID: PMC8052712
- DOI: 10.1186/s12883-021-02187-8
Prediction of survival in amyotrophic lateral sclerosis: a nationwide, Danish cohort study
Abstract
Introduction: Amyotrophic lateral sclerosis (ALS) is a progressive motor neuron disease with great heterogeneity. Biological prognostic markers are needed for the patients to plan future supportive treatment, palliative treatment, and end-of-life decisions. In addition, prognostic markers are greatly needed for the randomization in clinical trials.
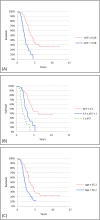
Objective: This study aimed to test the ALS Functional Rating Scale-Revised (ALSFRS-R) progression rate (ΔFS) as a prognostic marker of survival in a Danish ALS cohort.
Methods: The ALSFRS-R score at test date in association with duration of symptoms, from the onset of symptoms until test date, (defined as ΔFS') was calculated for 90 Danish patients diagnosed with either probable or definite sporadic ALS. Median survival time was then estimated from the onset of symptoms until primary endpoint (either death or tracheostomy). ΔFS' was subjected to survival analysis using Cox proportional hazards modelling, log-rank test, and Kaplan-Meier survival analysis.
Results and conclusions: Both ΔFS' and age was found to be strong predictors of survival of the Danish ALS cohort. Both variables are easily obtained at the time of diagnosis and could be used by clinicians and ALS patients to plan future supportive and palliative treatment. Furthermore, ΔFS', is a simple, prognostic marker that predicts survival in the early phase of disease as well as at later stages of the disease.
Keywords: ALSFRS-R slope; Amyotrophic lateral sclerosis; Median survival time; Prognostic biomarker.
Conflict of interest statement
None declared.
Figures
References
-
- Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, Nakanishi A. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS study group (phase III) J Neurol Sci. 1999;169(1–2):13–21. doi: 10.1016/S0022-510X(99)00210-5. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

