Palliative care in day-hospital for advanced cancer patients: a study protocol for a multicentre randomized controlled trial
- PMID: 33865379
- PMCID: PMC8053288
- DOI: 10.1186/s12904-021-00754-x
Palliative care in day-hospital for advanced cancer patients: a study protocol for a multicentre randomized controlled trial
Abstract
Background: Team-based and timely integrated palliative care is a gold standard of care in oncology, but issues concerning its optimal organization remain. Palliative Care in Day-Hospital (PCDH) could be one of the most efficient service model of palliative care to deliver interdisciplinary and multidimensional care addressing the complex supportive care needs of patients with advanced cancer. We hypothesize that, compared to conventional outpatient palliative care, PCDH allows the clinical benefits of palliative care to be enhanced.
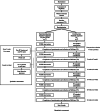
Methods/design: This study is a multicentre parallel group trial with stratified randomization. Patient management in PCDH will be compared to conventional outpatient palliative care. The inclusion criteria are advanced cancer patients referred to a palliative care team with an estimated life expectancy of more than 2 months and less than 1 year. The primary endpoint is health-related quality of life with deterioration-free survival based on the EORTC QLQ-C30 questionnaire. The secondary objectives are the following: increase in patient satisfaction with care using the EORTC PATSAT-C33 and OUT-PATSAT7 questionnaires, better understanding of the prognosis using the PTPQ questionnaire and advance care planning; decrease in the need for supportive care among relatives using the SCNS-P&C-F questionnaire, and reduction in end-of-life care aggressiveness. Patients will complete one to five questionnaires on a tablet before each monthly visit over 6 months and will be followed for 1 year. A qualitative study will take place, aiming to understand the specificity of palliative care management in PCDH. Cost-effectiveness, cost-utility and, an additional economic evaluation based on capability approach will be conducted from a societal point of view.
Discussion: The first strength of this study is that it combines the main relevant outcomes assessing integrated palliative care; patient quality of life and satisfaction; discussion of the prognosis and advance care planning, family well-being and end-of-life care aggressiveness. The second strength of the study is that it is a mixed-method study associating a qualitative analysis of the specificity of PCDH organization, with a medical-economic study to analyse the cost of care.
Trial registration: Name of the registry: IDRCB 2019-A03116-51 Trial registration number: NCT04604873 Date of registration: October 27, 2020 URL of trial registry record.
Keywords: Advanced cancer; End-of-life care; Palliative care; Quality of life; Study design.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Temel JS, Greer JA, El-Jawahri A, Pirl WF, Park ER, Jackson VA, Back AL, Kamdar M, Jacobsen J, Chittenden EH, Rinaldi SP, Gallagher ER, Eusebio JR, Li Z, Muzikansky A, Ryan DP. Effects of early integrated palliative Care in Patients with lung and GI Cancer: a randomized clinical trial. J Clin Oncol. 2017;35(8):834–841. doi: 10.1200/JCO.2016.70.5046. - DOI - PMC - PubMed
-
- Vanbutsele G, Pardon K, Van Belle S, Surmont V, De Laat M, Colman R, Eecloo K, Cocquyt V, Geboes K, Deliens L. Effect of early and systematic integration of palliative care in patients with advanced cancer: a randomised controlled trial. Lancet Oncol. 2018;19(3):394–404. doi: 10.1016/S1470-2045(18)30060-3. - DOI - PubMed
-
- Groenvold M, Petersen MA, Damkier A, Neergaard MA, Nielsen JB, Pedersen L, Sjøgren P, Strömgren AS, Vejlgaard TB, Gluud C, Lindschou J, Fayers P, Higginson IJ, Johnsen AT. Randomised clinical trial of early specialist palliative care plus standard care versus standard care alone in patients with advanced cancer: the Danish palliative care trial. Palliat Med. 2017;31(9):814–824. doi: 10.1177/0269216317705100. - DOI - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical