SARS-CoV-2 seropositivity and subsequent infection risk in healthy young adults: a prospective cohort study
- PMID: 33865504
- PMCID: PMC8049591
- DOI: 10.1016/S2213-2600(21)00158-2
SARS-CoV-2 seropositivity and subsequent infection risk in healthy young adults: a prospective cohort study
Abstract
Background: Whether young adults who are infected with SARS-CoV-2 are at risk of subsequent infection is uncertain. We investigated the risk of subsequent SARS-CoV-2 infection among young adults seropositive for a previous infection.
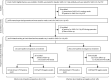
Methods: This analysis was performed as part of the prospective COVID-19 Health Action Response for Marines study (CHARM). CHARM included predominantly male US Marine recruits, aged 18-20 years, following a 2-week unsupervised quarantine at home. After the home quarantine period, upon arrival at a Marine-supervised 2-week quarantine facility (college campus or hotel), participants were enrolled and were assessed for baseline SARS-CoV-2 IgG seropositivity, defined as a dilution of 1:150 or more on receptor-binding domain and full-length spike protein ELISA. Participants also completed a questionnaire consisting of demographic information, risk factors, reporting of 14 specific COVID-19-related symptoms or any other unspecified symptom, and brief medical history. SARS-CoV-2 infection was assessed by PCR at weeks 0, 1, and 2 of quarantine and participants completed a follow-up questionnaire, which included questions about the same COVID-19-related symptoms since the last study visit. Participants were excluded at this stage if they had a positive PCR test during quarantine. Participants who had three negative swab PCR results during quarantine and a baseline serum serology test at the beginning of the supervised quarantine that identified them as seronegative or seropositive for SARS-CoV-2 then went on to basic training at Marine Corps Recruit Depot-Parris Island. Three PCR tests were done at weeks 2, 4, and 6 in both seropositive and seronegative groups, along with the follow-up symptom questionnaire and baseline neutralising antibody titres on all subsequently infected seropositive and selected seropositive uninfected participants (prospective study period).
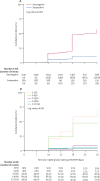
Findings: Between May 11, 2020, and Nov 2, 2020, we enrolled 3249 participants, of whom 3168 (98%) continued into the 2-week quarantine period. 3076 (95%) participants, 2825 (92%) of whom were men, were then followed up during the prospective study period after quarantine for 6 weeks. Among 189 seropositive participants, 19 (10%) had at least one positive PCR test for SARS-CoV-2 during the 6-week follow-up (1·1 cases per person-year). In contrast, 1079 (48%) of 2247 seronegative participants tested positive (6·2 cases per person-year). The incidence rate ratio was 0·18 (95% CI 0·11-0·28; p<0·001). Among seropositive recruits, infection was more likely with lower baseline full-length spike protein IgG titres than in those with higher baseline full-length spike protein IgG titres (hazard ratio 0·45 [95% CI 0·32-0·65]; p<0·001). Infected seropositive participants had viral loads that were about 10-times lower than those of infected seronegative participants (ORF1ab gene cycle threshold difference 3·95 [95% CI 1·23-6·67]; p=0·004). Among seropositive participants, baseline neutralising titres were detected in 45 (83%) of 54 uninfected and in six (32%) of 19 infected participants during the 6 weeks of observation (ID50 difference p<0·0001).
Interpretation: Seropositive young adults had about one-fifth the risk of subsequent infection compared with seronegative individuals. Although antibodies induced by initial infection are largely protective, they do not guarantee effective SARS-CoV-2 neutralisation activity or immunity against subsequent infection. These findings might be relevant for optimisation of mass vaccination strategies.
Funding: Defense Health Agency and Defense Advanced Research Projects Agency.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests DMP owned stock in Co-Diagnostics Inc during the conduct of the study. DS and FK have filed a patent regarding serological assays for SARS-CoV-2 and Icahn School of Medicine at Mount Sinai has founded a company to commercialise serological assays they developed. AGL, CG, DLW, HWC, DE, WDG, FJ, JM, EN, CKP, ESA, MS, VAS, RAL, SEL, PS, MT, and PS are military service members or government service employees. This work was prepared as part of their official duties. Title 17, US Code §105 provides that copyright protection under this title is not available for any work of the US Government. Title 17, US code §101 defines a US Government work as a work prepared by a military service member or employee of the US Government as part of that person's official duties. The views expressed in the article are those of the authors and do not necessarily express the official policy and position of the US Navy, the Department of Defense, the US Government or the institutions affiliated with the authors. AGL, YG, SV, CG, DLW, HWC, NAK, CAB, DE, M-CG, WDG, FJ, RAL, SEL, JM, NM, CMM, PB, SM, VDN, EN, CKP, ESA, AS-S, MS, VAS, MT, PS, RPT, AB, IR, and SCS declare no competing interests.
Figures
Comment in
-
SARS-CoV-2 reinfection in a closed setting: lessons for the community.Lancet Respir Med. 2021 Jul;9(7):675-677. doi: 10.1016/S2213-2600(21)00187-9. Epub 2021 Apr 15. Lancet Respir Med. 2021. PMID: 33865505 Free PMC article. No abstract available.
-
Viable virus shedding during SARS-CoV-2 reinfection.Lancet Respir Med. 2021 Jul;9(7):e56-e57. doi: 10.1016/S2213-2600(21)00219-8. Epub 2021 May 5. Lancet Respir Med. 2021. PMID: 33964243 Free PMC article. No abstract available.
References
-
- Stadlbauer D, Tan J, Jiang K, et al. Repeated cross-sectional sero-monitoring of SARS-CoV-2 in New York City. Nature. 2021;590:146–150. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

