Primate Spatial Memory Cells Become Tuned Early and Lose Tuning at Cell-Specific Times
- PMID: 33866356
- PMCID: PMC8328217
- DOI: 10.1093/cercor/bhab079
Primate Spatial Memory Cells Become Tuned Early and Lose Tuning at Cell-Specific Times
Abstract
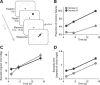
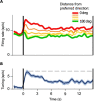
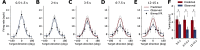
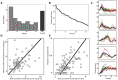
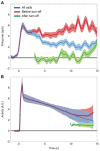
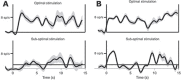
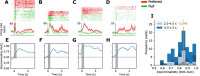
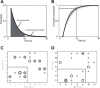
Working memory, the ability to maintain and transform information, is critical for cognition. Spatial working memory is particularly well studied. The premier model for spatial memory is the continuous attractor network, which posits that cells maintain constant activity over memory periods. Alternative models propose complex dynamics that result in a variety of cell activity time courses. We recorded from neurons in the frontal eye fields and dorsolateral prefrontal cortex of 2 macaques during long (5-15 s) memory periods. We found that memory cells turn on early after stimulus presentation, sustain activity for distinct and fixed lengths of time, then turn off and stay off for the remainder of the memory period. These dynamics are more complex than the dynamics of a canonical bump attractor network model (either decaying or nondecaying) but more constrained than the dynamics of fully heterogeneous memory models. We speculate that memory may be supported by multiple attractor networks working in parallel, with each network having its own characteristic mean turn-off time such that mnemonic resources are gradually freed up over time.
Keywords: frontal eye fields; macaque; prefrontal cortex; working memory.
© The Author(s) 2021. Published by Oxford University Press. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures

References
-
- Amit DJ. 1992. Modeling brain function: the world of attractor neural networks. Cambridge: Cambridge University Press.
-
- Amit DJ, Brunel N. 1997. Model of global spontaneous activity and local structured activity during delay periods in the cerebral cortex. Cereb Cortex. 7:237–252. - PubMed
-
- Baeg E, Kim Y, Huh K, Mook-Jung I, Kim H, Jung M. 2003. Dynamics of population code for working memory in the prefrontal cortex. Neuron. 40:177–188. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

