Transport-coupled ubiquitination of the borate transporter BOR1 for its boron-dependent degradation
- PMID: 33866370
- PMCID: PMC8136889
- DOI: 10.1093/plcell/koaa020
Transport-coupled ubiquitination of the borate transporter BOR1 for its boron-dependent degradation
Abstract
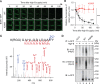
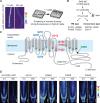
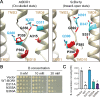
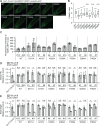
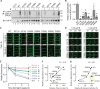
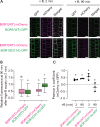
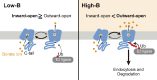
Plants take up and translocate nutrients through transporters. In Arabidopsis thaliana, the borate exporter BOR1 acts as a key transporter under boron (B) limitation in the soil. Upon sufficient-B supply, BOR1 undergoes ubiquitination and is transported to the vacuole for degradation, to avoid overaccumulation of B. However, the mechanisms underlying B-sensing and ubiquitination of BOR1 are unknown. In this study, we confirmed the lysine-590 residue in the C-terminal cytosolic region of BOR1 as the direct ubiquitination site and showed that BOR1 undergoes K63-linked polyubiquitination. A forward genetic screen identified that amino acid residues located in vicinity of the substrate-binding pocket of BOR1 are essential for the vacuolar sorting. BOR1 variants that lack B-transport activity showed a significant reduction of polyubiquitination and subsequent vacuolar sorting. Coexpression of wild-type (WT) and a transport-defective variant of BOR1 in the same cells showed degradation of the WT but not the variant upon sufficient-B supply. These findings suggest that polyubiquitination of BOR1 relies on its conformational transition during the transport cycle. We propose a model in which BOR1, as a B transceptor, directly senses the B concentration and promotes its own polyubiquitination and vacuolar sorting for quick and precise maintenance of B homeostasis.
© The Author(s) 2020. Published by Oxford University Press on behalf of American Society of Plant Biologists.
Figures


References
-
- Clough SJ, Bent AF (1998) Floral dip: a simplified method forAgrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

