A pharmacokinetics study of proposed bevacizumab biosimilar MYL-1402O vs EU-bevacizumab and US-bevacizumab
- PMID: 33866430
- PMCID: PMC8800899
- DOI: 10.1007/s00432-021-03628-0
A pharmacokinetics study of proposed bevacizumab biosimilar MYL-1402O vs EU-bevacizumab and US-bevacizumab
Abstract
Purpose: Bevacizumab is a recombinant humanized monoclonal antibody that inhibits vascular endothelial growth factor-specific angiogenesis in some cancers. MYL-1402O is a proposed bevacizumab biosimilar.
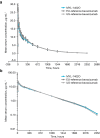
Methods: The primary objective of this single-center, randomized, double-blind, three-arm, parallel-group, phase 1 study in healthy male volunteers was to evaluate bioequivalence of MYL-1402O to EU and US-reference bevacizumab, and EU-reference bevacizumab to US-reference bevacizumab. The primary pharmacokinetic parameter was area under the serum concentration-time curve from 0 extrapolated to infinity (AUC0-∞). Pharmacokinetic parameters were analyzed using general linear models of analysis of variance. Secondary endpoints included safety and tolerability.
Results: Of 111 enrolled subjects, 110 were included in the pharmacokinetic analysis (MYL-1402O, n = 37; EU-reference bevacizumab, n = 36; US-reference bevacizumab, n = 37). Bioequivalence was demonstrated between MYL-1402O and EU-reference bevacizumab, MYL-1402O and US-reference bevacizumab, and between EU- and US-reference bevacizumab where least squares mean ratios of AUC0-∞ were close to 1, and 90% CIs were within the equivalence range (0.80-1.25). Secondary pharmacokinetic parameters (AUC from 0 to time of last quantifiable concentration [AUC0-t], peak serum concentration [Cmax], time to Cmax, elimination rate constant, and elimination half-life) were also comparable, with 90% CIs for ratios of AUC0-t and Cmax within 80-125%. Treatment-emergent adverse events were similar across all three treatment groups and were consistent with clinical data for bevacizumab.
Conclusion: MYL-1402O was well tolerated and demonstrated pharmacokinetic and safety profiles similar to EU-reference bevacizumab and US-reference bevacizumab in healthy male volunteers. No new significant safety issues emerged (ClinicalTrials.gov, NCT02469987; ClinicalTrialsRegister.eu EudraCT, 2014-005621-12; June 12, 2015).
Keywords: Bioequivalence; Cancer; Monoclonal antibody; Pharmacokinetics; Phase 1.
© 2021. The Author(s).
Conflict of interest statement
M Hummel, A Shaw, MS Liu, and A Barve are employees of Viatris Inc and may hold stock with the company. T Bosje was an employee of PRA Health Sciences during the study and has since retired, and has nothing to disclose. M Kothekar was an employee of Biocon Research Ltd at the time of the study and may hold stock with the company. M Socinski has nothing to disclose. CF Waller is a consultant/advisory board member for Viatris Inc.
Figures

References
-
- André T, Kotelevets L, Vaillant JC, Coudray AM, Weber L, Prévot S, Parc R, Gespach C, Chastre E (2000) Vegf, Vegf-B, Vegf-C and their receptors KDR, FLT-1 and FLT-4 during the neoplastic progression of human colonic mucosa. Int J Cancer 86:174–181. 10.1002/(sici)1097-0215(20000415)86:2<174::aid-ijc5>3.0.co;2-e - PubMed
-
- Avastin [package insert] (2019) Genentech, Inc, South San Francisco
-
- Avastin [summary of product characteristics] (2020) Roche Pharma AG, Grenzach-Wyhlen
-
- Beniwal S, Kothekar M, Loganathan S, Vishweswaramurthy A, Marwah A, Pennella E, Sengupta N (2017) Comparative pharmacokinetics of a proposed biosimilar bevacizumab Bmab-100 and reference product bevacizumab in a multicentre double blind randomized clinical trial in metastatic colorectal carcinoma (mCRC) patients. Ann Oncol. 28(suppl 10). 10.1093/annonc/mdx659.016
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

