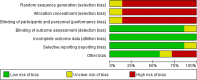
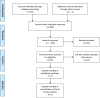
Meta-analysis of the benefit of hypomethylating agents before allogeneic hematopoietic stem cell transplantation in myelodysplastic syndromes
- PMID: 33866494
- PMCID: PMC8505317
- DOI: 10.1007/s10238-021-00712-0
Meta-analysis of the benefit of hypomethylating agents before allogeneic hematopoietic stem cell transplantation in myelodysplastic syndromes
Abstract
Hypomethylating agents (HMAs) are effective therapies in myelodysplastic syndromes (MDS), but allogeneic hematopoietic stem cell transplantation (allo-HSCT) is the only way to cure MDS. According to the current literature, it is difficult to confirm whether HMAs bridging therapy is beneficial for MDS patients receiving allo-HSCT. Therefore, we tried to evaluate the effect of HMAs on long-term survival of the MDS patients. Databases, including PubMed, Embase Ovid, and the Cochrane Library, were searched for studies published up to January 10, 2021. Patients who accepted HMAs bridging to allo-HSCT were defined as experimental group, while patients who received the best supportive care (BSC) before allo-HSCT were control group. Overall survival (OS) was the primary end point. Seven studies were included in the final analysis. The final results showed no OS differences between patients accepted HMAs before allo-HSCT and those received BSC (HR = 0.86, 95% CI: 0.64-1.15, p = 0.32), indicating that MDS patients' long-term survival did not benefit from HMAs bridging therapy before allo-HSCT. This conclusion needs to be further verified by a large number of prospective randomized controlled trials, which have guiding significance for the treatment of MDS patients.
Keywords: Allogeneic hematopoietic stem cell transplantation; Hypomethylating agents; Meta-analysis; Myelodysplastic syndromes.
© 2021. The Author(s).
Conflict of interest statement
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Figures
References
-
- Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Finelli C, Giagounidis A, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009;10(3):223–232. doi: 10.1016/S1470-2045(09)70003-8. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous