Immunoglobulin, nucleos(t)ide analogues and hepatitis B virus recurrence after liver transplant: A meta-analysis
- PMID: 33866547
- PMCID: PMC8365701
- DOI: 10.1111/eci.13575
Immunoglobulin, nucleos(t)ide analogues and hepatitis B virus recurrence after liver transplant: A meta-analysis
Abstract
Background: Prophylaxis with hepatitis B immunoglobulin (HBIG) represents an efficient strategy for reducing the risk of hepatitis B virus (HBV) recurrence after liver transplantation (LT). Unfortunately, the long-term use of HBIG presents high costs. Therefore, the use of prophylaxis based only on nucleos(t)ide analogues (NUC) has been recently postulated. The present meta-analysis aimed to evaluate the impact of HBIG ± NUC vs HBIG alone or NUC alone in post-LT HBV recurrence prophylaxis.
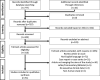
Materials and methods: A systematic literature search was performed using PubMed and Cochrane databases. The primary outcome investigated was the HBV recurrence after LT. Three analyses were done comparing the effect of (a) HBIG + NUC vs HBIG alone; (b) HBIG+NUC vs NUC alone; and (c) HBIG alone vs NUC alone. Sub-analyses were also performed investigating the effect of low and high genetic barrierto-recurrence NUC.
Results: Fifty-one studies were included. The summary OR (95%CI) showed a decreased risk with the combination of HBIG + NUC vs HBIG alone for HBV recurrence, being 0.36 (95% CI = 0.22-0.61; P < .001). HBIG + NUC combined treatment reduced HBV reappearance respect to NUC alone (OR = 0.22; 95% CI = 0.16-0.30; P < .0001). Similarly, HBIG alone was significantly better than NUC alone in preventing HBV recurrence (OR = 0.20; 95% CI = 0.09-0.44; P < .0001).
Conclusions: Prophylaxis with HBIG is relevant in preventing post-LT HBV recurrence. Its combination with NUC gives the best results in terms of protection. The present results should be considered in light of the fact that also old studies based on lamivudine use were included. Studies exploring in detail high genetic barrier-to-recurrence NUC and protocols with definite use of HBIG are needed.
Keywords: adefovir; entecavir; lamivudine; liver transplantation; nucleos(t)ide analogues; prophylaxis.
© 2021 The Authors. European Journal of Clinical Investigation published by John Wiley & Sons Ltd on behalf of Stichting European Society for Clinical Investigation Journal Foundation.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures




References
-
- Mosley JW. Editorial: the HBV carrier–a new kind of leper? N Engl J Med. 1975;292:477‐478. - PubMed
-
- Polaris Observatory Collaborators . Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol. 2018;3:383‐403. - PubMed
-
- Kennedy M, Alexopoulos SP. Hepatitis B virus infection and liver transplantation. Curr Opin Organ Transplant. 2010;15:310‐315. - PubMed
-
- Samuel D, Bismuth A, Mathieu D, et al. Passive immunoprophylaxis after liver transplantation in HBsAg‐positive patients. Lancet. 1991;337:813‐815. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

