16α-Bromoepiandrosterone as a new candidate for experimental diabetes-tuberculosis co-morbidity treatment
- PMID: 33866550
- PMCID: PMC8274213
- DOI: 10.1111/cei.13603
16α-Bromoepiandrosterone as a new candidate for experimental diabetes-tuberculosis co-morbidity treatment
Abstract
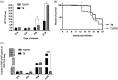
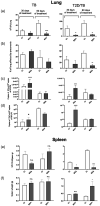
Tuberculosis (TB) is the leading cause of death from a single bacterial infectious agent and is one of the most relevant issues of public health. Another pandemic disease is type II diabetes mellitus (T2D) that is estimated to affect half a billion people in the world. T2D is directly associated with obesity and a sedentary lifestyle and is frequently associated with immunosuppression. Immune dysfunction induced by hyperglycemia increases infection frequency and severity. Thus, in developing countries the T2D/TB co-morbidity is frequent and represents one of the most significant challenges for the health-care systems. Several immunoendocrine abnormalities are occurring during the chronic phase of both diseases, such as high extra-adrenal production of active glucocorticoids (GCs) by the activity of 11-β-hydroxysteroid dehydrogenase type 1 (11-βHSD1). 11-βHSD1 catalyzes the conversion of inactive cortisone to active cortisol or corticosterone in lungs and liver, while 11-β-hydroxysteroid dehydrogenase type 2 (11-βHSD2) has the opposite effect. Active GCs have been related to insulin resistance and suppression of Th1 responses, which are deleterious factors in both T2D and TB. The anabolic adrenal hormone dehydroepiandrosterone (DHEA) exerts antagonistic effects on GC signaling in immune cells and metabolic tissues; however, its anabolic effects prohibit its use to treat immunoendocrine diseases. 16α-bromoepiandrosterone (BEA) is a water miscible synthetic sterol related to DHEA that lacks an anabolic effect while amplifying the immune and metabolic properties with important potential therapeutic uses. In this work, we compared the expression of 11-βHSD1 and the therapeutic efficacy of BEA in diabetic mice infected with tuberculosis (TB) (T2D/TB) with respect to non-diabetic TB-infected mice (TB). T2D was induced by feeding mice with a high-fat diet and administering a single low-dose of streptozotocin. After 4 weeks of T2D establishment, mice were infected intratracheally with a high-dose of Mycobacterium tuberculosis strain H37Rv. Then, mice were treated with BEA three times a week by subcutaneous and intratracheal routes. Infection with TB increased the expression of 11-βHSD1 and corticosterone in the lungs and liver of both T2D/TB and TB mice; however, T2D/TB mice developed a more severe lung disease than TB mice. In comparison with untreated animals, BEA decreased GC and 11-βHSD1 expression while increasing 11-βHSD2 expression. These molecular effects of BEA were associated with a reduction in hyperglycemia and liver steatosis, lower lung bacillary loads and pneumonia. These results uphold BEA as a promising effective therapy for the T2D/TB co-morbidity.
Keywords: 11-βHSD1; BEA; active glucocorticoids; central nervous system; colony-forming units; diabetes-tuberculosis co-morbidity; immunotherapy.
© 2021 British Society for Immunology.
Conflict of interest statement
We declare no conflicts of interest during the course of this work.
Figures




References
-
- Meenakshi P, Ramya S, Lavanya J, Vijayalakshmi V, Sumanlatha G. Effect of IFN‐c, IL‐12 and IL‐10 cytokine production and mRNA expression in tuberculosis patients with diabetes mellitus and their household contacts. Cytokine 2016;81:127–36. https://doi.org/10.1016/j.cyto.2016.03.009. - DOI - PubMed
-
- Rook WG. Th2 cytokines in susceptibility to tuberculosis. Curr Mol Med. 2007;7:327–37. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

