Artificial Intelligence for Unstructured Healthcare Data: Application to Coding of Patient Reporting of Adverse Drug Reactions
- PMID: 33866552
- PMCID: PMC8359992
- DOI: 10.1002/cpt.2266
Artificial Intelligence for Unstructured Healthcare Data: Application to Coding of Patient Reporting of Adverse Drug Reactions
Abstract
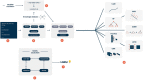
Adverse drug reaction (ADR) reporting is a major component of drug safety monitoring; its input will, however, only be optimized if systems can manage to deal with its tremendous flow of information, based primarily on unstructured text fields. The aim of this study was to develop an automated system allowing to code ADRs from patient reports. Our system was based on a knowledge base about drugs, enriched by supervised machine learning (ML) models trained on patients reporting data. To train our models, we selected all cases of ADRs reported by patients to a French Pharmacovigilance Centre through a national web-portal between March 2017 and March 2019 (n = 2,058 reports). We tested both conventional ML models and deep-learning models. We performed an external validation using a dataset constituted of a random sample of ADRs reported to the Marseille Pharmacovigilance Centre over the same period (n = 187). Here, we show that regarding area under the curve (AUC) and F-measure, the best model to identify ADRs was gradient boosting trees (LGBM), with an AUC of 0.93 (0.92-0.94) and F-measure of 0.72 (0.68-0.75). This model was run for external validation showing an AUC of 0.91 and a F-measure of 0.58. We evaluated an artificial intelligence pipeline that was found able to learn how to identify correctly ADRs from unstructured data. This result allowed us to start a new study using more data to further improve our performance and offer a tool that is useful in practice to efficiently manage drug safety information.
© 2021 The Authors. Clinical Pharmacology & Therapeutics published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
L.L., J.J., A.B., and C.G. were employed by Synapse Medicine at the time this research was conducted or hold stock/stock options therein. All other authors declared no competing interests.
Figures


References
-
- Sheikh, A., Jha, A., Cresswell, K., Greaves, F. & Bates, D.W. Adoption of electronic health records in UK hospitals: lessons from the USA. Lancet 384, 8–9 (2014). - PubMed
-
- Kish, L.J. & Topol, E.J. Unpatients—why patients should own their medical data. Nat. Biotechnol. 33, 921–924 (2015). - PubMed
-
- Cambridge dictionary <https://dictionary.cambridge.org/fr/dictionnaire/anglais/artificial‐inte...>. Accessed April 9, 2021.
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

