Epigenetics drive the evolution of sex chromosomes in animals and plants
- PMID: 33866802
- PMCID: PMC8059572
- DOI: 10.1098/rstb.2020.0124
Epigenetics drive the evolution of sex chromosomes in animals and plants
Abstract
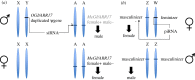
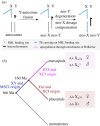
We review how epigenetics affect sex chromosome evolution in animals and plants. In a few species, sex is determined epigenetically through the action of Y-encoded small RNAs. Epigenetics is also responsible for changing the sex of individuals through time, even in species that carry sex chromosomes, and could favour species adaptation through breeding system plasticity. The Y chromosome accumulates repeats that become epigenetically silenced which leads to an epigenetic conflict with the expression of Y genes and could accelerate Y degeneration. Y heterochromatin can be lost through ageing, which activates transposable elements and lowers male longevity. Y chromosome degeneration has led to the evolution of meiotic sex chromosome inactivation in eutherians (placentals) and marsupials, and dosage compensation mechanisms in animals and plants. X-inactivation convergently evolved in eutherians and marsupials via two independently evolved non-coding RNAs. In Drosophila, male X upregulation by the male specific lethal (MSL) complex can spread to neo-X chromosomes through the transposition of transposable elements that carry an MSL-binding motif. We discuss similarities and possible differences between plants and animals and suggest future directions for this dynamic field of research. This article is part of the theme issue 'How does epigenetics influence the course of evolution?'
Keywords: X chromosome inactivation; X upregulation; Y degeneration; Y toxicity; imprinting; meiotic sex chromosome inactivation.
Figures

Similar articles
-
The epigenome of evolving Drosophila neo-sex chromosomes: dosage compensation and heterochromatin formation.PLoS Biol. 2013 Nov;11(11):e1001711. doi: 10.1371/journal.pbio.1001711. Epub 2013 Nov 12. PLoS Biol. 2013. PMID: 24265597 Free PMC article.
-
Dosage compensation evolution in plants: theories, controversies and mechanisms.Philos Trans R Soc Lond B Biol Sci. 2022 May 9;377(1850):20210222. doi: 10.1098/rstb.2021.0222. Epub 2022 Mar 21. Philos Trans R Soc Lond B Biol Sci. 2022. PMID: 35306896 Free PMC article. Review.
-
Weird mammals provide insights into the evolution of mammalian sex chromosomes and dosage compensation.J Genet. 2015 Dec;94(4):567-74. doi: 10.1007/s12041-015-0572-3. J Genet. 2015. PMID: 26690510 Review.
-
Impact of repetitive DNA on sex chromosome evolution in plants.Chromosome Res. 2015 Sep;23(3):561-70. doi: 10.1007/s10577-015-9496-2. Chromosome Res. 2015. PMID: 26474787
-
Sex-specific embryonic gene expression in species with newly evolved sex chromosomes.PLoS Genet. 2014 Feb 13;10(2):e1004159. doi: 10.1371/journal.pgen.1004159. eCollection 2014 Feb. PLoS Genet. 2014. PMID: 24550743 Free PMC article.
Cited by
-
The Role of Transposable Elements in Sexual Development.Front Behav Neurosci. 2022 Jul 7;16:923732. doi: 10.3389/fnbeh.2022.923732. eCollection 2022. Front Behav Neurosci. 2022. PMID: 35874645 Free PMC article. Review.
-
Predator-induced transgenerational plasticity of parental care behaviour in male three-spined stickleback fish across two generations.Proc Biol Sci. 2024 Jan 10;291(2014):20232582. doi: 10.1098/rspb.2023.2582. Epub 2024 Jan 10. Proc Biol Sci. 2024. PMID: 38196352 Free PMC article.
-
Y chromosome toxicity does not contribute to sex-specific differences in longevity.Nat Ecol Evol. 2023 Aug;7(8):1245-1256. doi: 10.1038/s41559-023-02089-7. Epub 2023 Jun 12. Nat Ecol Evol. 2023. PMID: 37308701 Free PMC article.
-
Chromatin Landscape Is Associated With Sex-Biased Expression and Drosophila-Like Dosage Compensation of the Z Chromosome in Artemia franciscana.Mol Biol Evol. 2025 Apr 30;42(5):msaf085. doi: 10.1093/molbev/msaf085. Mol Biol Evol. 2025. PMID: 40202086 Free PMC article.
-
How does epigenetics influence the course of evolution?Philos Trans R Soc Lond B Biol Sci. 2021 Jun 7;376(1826):20200111. doi: 10.1098/rstb.2020.0111. Epub 2021 Apr 19. Philos Trans R Soc Lond B Biol Sci. 2021. PMID: 33866814 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

