Manipulating the solution environment to control the surface roughness of elastin-based polymer coatings
- PMID: 33866852
- PMCID: PMC8516518
- DOI: 10.1177/08853282211010302
Manipulating the solution environment to control the surface roughness of elastin-based polymer coatings
Abstract
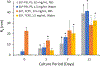
Elastin-like polypeptides (ELP) have been used as a genetically-engineered, biocompatible substitute for elastin. Cell culture coatings prepared using ELP conjugated to low molecular weight polyethyleneimine (PEI) entices cells to form three-dimensional cellular aggregates that mimic their in vivo counterparts. This study seeks to control the deposition of the ELP and ELP-PEI molecules to control the roughness of the final coatings. The two polymers were coated onto three different substrates (glass, polystyrene, tissue-culture polystyrene) and the solution environment was altered by changing the polymer concentration (0.5, 1.0, 1.5 mg/mL) and/or salt concentration (None, 0.2 M phosphate buffered saline) for a total of 36 conditions. Atomic force microscopy (AFM) was used to measure the average roughness (Ra) of the samples and found that ELP coated samples had a higher Ra than their ELP-PEI counterparts. The coatings were tested for stability by performing cell culture media changes every three days for 11 days. AFM showed that the average roughness of the tested samples increased with each media change. To address this, the surfaces were crosslinked using hexamethyl diisocyanate, which minimized the change in surface roughness even when subjected to an intense sonication process. This study provides parameters to achieve elastin-based coatings with controlled roughness that can be used to support stable, long-term in vitro cell culture.
Keywords: Elastin-like polypeptide; atomic force microscopy; coating; roughness.
Figures





References
-
- Urry DW. Entropic elastic processes in protein mechanisms. I. Elastic structure due to an inverse temperature transition and elasticity due to internal chain dynamics. Journal of Protein Chemistry. 1988;7:1–34. - PubMed
-
- Urry DW. Free energy transduction in polypeptides and proteins based on inverse temperature transitions. Progress in Biophysics and Molecular Biology. 1992;57:23–57. - PubMed
-
- Urry DW. Physical chemistry of biological free energy transduction as demonstrated by elastic protein-based polymers. Journal of Physical Chemistry: 1997;101:11007–11028.
-
- Meyer DE, Chilkoti A. Quantification of the effects of chain length and concentration on the thermal behavior of elastin-like polypeptides. Biomacromolecules. 2004;5:846–851. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

