The role of procalcitonin results in antibiotic decision-making in coronavirus disease 2019 (COVID-19)
- PMID: 33866995
- PMCID: PMC8485015
- DOI: 10.1017/ice.2021.175
The role of procalcitonin results in antibiotic decision-making in coronavirus disease 2019 (COVID-19)
Abstract
Objective: To evaluate the role of procalcitonin (PCT) results in antibiotic decisions for COVID-19 patients at hospital presentation.
Design, setting, and participants: Multicenter retrospective observational study of patients ≥18 years hospitalized due to COVID-19 at the Johns Hopkins Health system. Patients who were transferred from another facility with >24 hours stay and patients who died within 48 hours of hospitalization were excluded.
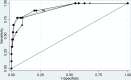
Methods: Elevated PCT values were determined based on each hospital's definition. Antibiotic therapy and PCT results were evaluated for patients with no evidence of bacterial community-acquired pneumonia (bCAP) and patients with confirmed, probable, or possible bCAP. The added value of PCT testing to clinical criteria in detecting bCAP was evaluated using receiving operating curve characteristics (ROC).
Results: Of 962 patients, 611 (64%) received a PCT test. ROC curves for clinical criteria and clinical criteria plus PCT test were similar (at 0.5 ng/mL and 0.25 ng/mL). By bCAP group, median initial PCT values were 0.58 ng/mL (interquartile range [IQR], 0.24-1.14), 0.23 ng/mL (IQR, 0.1-0.63), and 0.15 ng/mL (IQR, 0.09-0.35) for proven/probable, possible, and no bCAP groups, respectively. Among patients without bCAP, an elevated PCT level was associated with 1.8 additional days of CAP therapy (95% CI, 1.01-2.75; P < .01) compared to patients with a negative PCT result after adjusting for potential confounders. Duration of CAP therapy was similar between patients without a PCT test ordered and a low PCT level for no bCAP and possible bCAP groups.
Conclusions: PCT results may be abnormal in COVID-19 patients without bCAP and may result in receipt of unnecessary antibiotics.
Figures
References
-
- Melendi GA, Laham FR, Monsalvo AC, et al.Cytokine profiles in the respiratory tract during primary infection with human metapneumovirus, respiratory syncytial virus, or influenza virus in infants. Pediatrics 2007;120:e410–e415. - PubMed
-
- Gilbert DN.Procalcitonin as a biomarker in respiratory tract infection. Clin Infect Dis 2011;52 suppl4:S346–S350. - PubMed
-
- Schuetz P, Christ-Crain M, Muller B.Biomarkers to improve diagnostic and prognostic accuracy in systemic infections. Curr Opin Crit Care 2007;13:578–585. - PubMed
-
- Huang DT, Yealy DM, Angus DC, the Pro ACTI. Procalcitonin-guided antibiotic use. N Engl J Med 2018;379:1973. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

