Efficacy and safety of Lianhuaqingwen capsules, a repurposed Chinese herb, in patients with coronavirus disease 2019: A multicenter, prospective, randomized controlled trial
- PMID: 33867046
- PMCID: PMC7229744
- DOI: 10.1016/j.phymed.2020.153242
Efficacy and safety of Lianhuaqingwen capsules, a repurposed Chinese herb, in patients with coronavirus disease 2019: A multicenter, prospective, randomized controlled trial
Erratum in
-
Efficacy and safety of Lianhua Qingwen capsules, a repurposed Chinese herb, in patients with Coronavirus disease 2019: A multicenter, prospective, randomized controlled trial [Phytomedicine 85 (2021) 153242].Phytomedicine. 2022 Jan;94:153800. doi: 10.1016/j.phymed.2021.153800. Epub 2021 Oct 9. Phytomedicine. 2022. PMID: 34775361 Free PMC article. No abstract available.
Abstract
Background: Coronavirus disease 2019 (Covid-19) has resulted in a global outbreak. Few existing targeted medications are available. Lianhuaqingwen (LH) capsule, a repurposed marketed Chinese herb product, has been proven effective for influenza.
Purpose: To determine the safety and efficacy of LH capsule in patients with Covid-19.
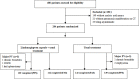
Methods: We did a prospective multicenter open-label randomized controlled trial on LH capsule in confirmed cases with Covid-19. Patients were randomized to receive usual treatment alone or in combination with LH capsules (4 capsules, thrice daily) for 14 days. The primary endpoint was the rate of symptom (fever, fatigue, coughing) recovery.
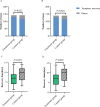
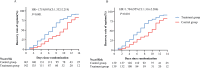
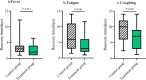
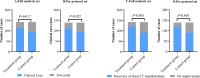
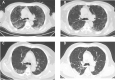
Results: We included 284 patients (142 each in treatment and control group) in the full-analysis set. The recovery rate was significantly higher in treatment group as compared with control group (91.5% vs. 82.4%, p = 0.022). The median time to symptom recovery was markedly shorter in treatment group (median: 7 vs. 10 days, p < 0.001). Time to recovery of fever (2 vs. 3 days), fatigue (3 vs. 6 days) and coughing (7 vs. 10 days) was also significantly shorter in treatment group (all p < 0.001). The rate of improvement in chest computed tomographic manifestations (83.8% vs. 64.1%, p < 0.001) and clinical cure (78.9% vs. 66.2%, p = 0.017) was also higher in treatment group. However, both groups did not differ in the rate of conversion to severe cases or viral assay findings (both p > 0.05). No serious adverse events were reported.
Conclusion: In light of the safety and effectiveness profiles, LH capsules could be considered to ameliorate clinical symptoms of Covid-19.
Keywords: Coronavirus disease 2019; Lianhuaqingwen Capsule; Symptom recovery; conversion rate.
Copyright © 2020. Published by Elsevier GmbH.
Conflict of interest statement
None.
Figures
References
-
- Chai X., Hu L., Zhang Y., Han W., Lu Z., Ke A., Zhou J., Shi G., Fang N., Fan J., Cai J., Fan J., Lan F. Specific ACE2 expression in cholangiocytes May cause liver damage after 2019-nCoV infection. BioRxiv. 2020
-
- Cheng D., Li Y. Clinical analysis and typical case report of Lianhua qingwen granule in treating 54 cases of new coronavirus pneumonia. World J. Tradit. Chin. Med. 2020;15:150–154.
-
- National Health Commission & State Administration of Traditional Chinese Medicine. Diagnosis and treatment protocol for coronavirus pneumonia (Trial version 7). March 3rd, 2020.
-
- Chinese Academy Of Military Hospital Chinese academy of military medical sciences and Beijing Ditan hospital have confirmed anti-H1N1 influenza virus: Chinese medicine Lianhua Qingwen capsule has made a major breakthrough. J. Chin. Prescrip. Drugs. 2009;9:41.
-
- Cui W., Jin X., Zhang Y. Effects of Lianhua Qingwen capsule on IKK / IκB / NF-κB signaling pathway in mice with acute lung injury induced by lipopolysaccharide. Chin. J. Pharm. Toxicol. 2015;37:953–958.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

