Timing of surgery in patients with partial response or stable disease after neoadjuvant chemotherapy for advanced ovarian cancer
- PMID: 33867146
- PMCID: PMC8165031
- DOI: 10.1016/j.ygyno.2021.04.012
Timing of surgery in patients with partial response or stable disease after neoadjuvant chemotherapy for advanced ovarian cancer
Abstract
Objective: The ideal number of neoadjuvant chemotherapy (NACT) cycles prior to interval tumor-reductive surgery (iTRS) for advanced ovarian cancer is poorly defined. We sought to assess survival stratified by number of NACT cycles and residual disease following iTRS in patients with advanced ovarian cancer with partial response (PR) or stable disease (SD) following 3-4 cycles of NACT.
Methods: We retrospectively identified patients with advanced high-grade ovarian cancer (diagnosed 2/1/2013 to 2/1/2018) who received at least 3 cycles of NACT and iTRS and had a PR or SD. The population was divided into four groups based on the number of NACT cycles prior to iTRS and residual disease status after (CGR [complete gross residual] or incomplete resection [any amount of residual disease]): 1) 3-4 NACT cycles/CGR, 2) 3-4 NACT cycles/incomplete resection, 3) > 4 cycles/CGR, and 4) >4 cycles/incomplete resection. Overall survival (OS) and progression-free survival (PFS) were estimated using a Kaplan-Meier product-limit estimator and modeled using univariable and multivariable Cox proportional hazards analysis.
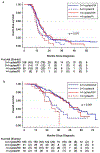
Results: The cohort consisted of 265 patients with advanced high-grade ovarian cancer with a median age at diagnosis of 65 years. Most were White (87%), had serous histology (89%), and stage IV disease (57%), with an overall CGR rate of 81%. In a multivariable analysis receipt of >4 NACT cycles was not associated with worse PFS or OS (adjusted hazard ratio [aHR] 1.02, 95% CI 0.74-1.42; aHR 1.12, 95% CI, 0.73-1.72 respectively) than was receipt of 3-4 cycles. Any amount of residual disease was associated with worse PFS and OS regardless of the number of NACT cycles (aHR 1.56, 95% CI 1.09-2.22; aHR 2.38, 95% CI 1.52-3.72 respectively).
Conclusions: Residual disease was associated with worse survival outcomes regardless of the number of NACT cycles in patients with PR or SD after NACT for advanced high-grade ovarian cancer. These data suggest that the ability to achieve CGR should take precedence in decision-making regarding the timing of surgery.
Keywords: Interval tumor reductive surgery; Neoadjuvant chemotherapy; Ovarian cancer.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest R.N., K.H.L, and J.A.R-H have nothing to disclose. N.D·F reports personal fees from Tesaro, personal fees from BMS/Pfizer, personal fees from Glaxo Smith Kline, outside the submitted work; B.M.F. and L.A.M report grants from NIH, during the conduct of the study. A.K.S reports grants from NIH Grants during the conduct of the study as well as consulting fees from Merck and Kiyatec, shareholder position in Biopath, other from Kiyatec, and research funding from M-Trap, outside the submitted work.
Figures
Similar articles
-
3-4 Cycles versus 6 Cycles Neoadjuvant Chemotherapy in Advanced-Stage Epithelial Ovarian Cancer: Survival Is Not Determined by the Number of Neoadjuvant Chemotherapy Cycles.Chemotherapy. 2024;69(2):122-132. doi: 10.1159/000535755. Epub 2023 Dec 19. Chemotherapy. 2024. PMID: 38113873
-
Primary debulking surgery versus primary neoadjuvant chemotherapy for high grade advanced stage ovarian cancer: comparison of survivals.Radiol Oncol. 2018 Sep 11;52(3):307-319. doi: 10.2478/raon-2018-0030. Radiol Oncol. 2018. PMID: 30210049 Free PMC article.
-
Prognostic impact of microscopic residual disease after neoadjuvant chemotherapy in patients undergoing interval debulking surgery for advanced ovarian cancer.Arch Gynecol Obstet. 2025 Feb;311(2):429-436. doi: 10.1007/s00404-024-07775-w. Epub 2024 Oct 13. Arch Gynecol Obstet. 2025. PMID: 39397086 Free PMC article.
-
Uptake and Outcomes of Neoadjuvant Chemotherapy Among US Patients With Less Common Epithelial Ovarian Carcinomas.JAMA Netw Open. 2023 Jun 1;6(6):e2318602. doi: 10.1001/jamanetworkopen.2023.18602. JAMA Netw Open. 2023. PMID: 37326992 Free PMC article.
-
Neoadjuvant Chemotherapy in Advanced Ovarian Cancer: A Single-Institution Experience and a Review of the Literature.Oncology. 2016;91(4):211-216. doi: 10.1159/000447743. Epub 2016 Aug 3. Oncology. 2016. PMID: 27487241 Review.
Cited by
-
Investigating the effect of optimal cytoreduction in the context of platinum sensitivity in high-grade serous ovarian cancer.Acta Obstet Gynecol Scand. 2022 Oct;101(10):1085-1092. doi: 10.1111/aogs.14415. Epub 2022 Jul 2. Acta Obstet Gynecol Scand. 2022. PMID: 35778930 Free PMC article.
-
Optimal number of neoadjuvant chemotherapy cycles prior to interval debulking surgery in advanced epithelial ovarian cancer: a systematic review and meta-analysis of progression-free survival and overall survival.J Gynecol Oncol. 2023 Nov;34(6):e82. doi: 10.3802/jgo.2023.34.e82. Epub 2023 Sep 5. J Gynecol Oncol. 2023. PMID: 37743060 Free PMC article.
-
Primary or Interval Debulking Surgery in Advanced Ovarian Cancer: a Personalized Decision-a Literature Review.Curr Oncol Rep. 2022 Dec;24(12):1661-1668. doi: 10.1007/s11912-022-01318-9. Epub 2022 Aug 15. Curr Oncol Rep. 2022. PMID: 35969358 Review.
-
The association of the chemotherapy response score and homologous recombination deficiency in patients undergoing interval tumor reductive surgery following neoadjuvant chemotherapy.Int J Gynecol Cancer. 2025 Jan 6:ijgc-2024-005893. doi: 10.1136/ijgc-2024-005893. Online ahead of print. Int J Gynecol Cancer. 2025. PMID: 39414313 Free PMC article.
References
-
- National Cancer Institute: Surveillance, epidemiology, and end results program. Cancer stat facts: ovarian cancer, (n.d.). https://seer.cancer.gov/statfacts/html/ovary.html (accessed January 25, 2021).
-
- Knisely AT, Clair CM St., Hou JY, Collado FK, Hershman DL, Wright JD, Melamed A, Trends in Primary Treatment and Median Survival Among Women With Advanced-Stage Epithelial Ovarian Cancer in the US From 2004 to 2016, JAMA Netw. Open 3 (2020) e2017517. doi:10.1001/jamanetworkopen.2020.17517. - DOI - PMC - PubMed
-
- Vergote I, Tropé CG, Amant F, Kristensen GB, Ehlen T, Johnson N, Verheijen RHM, van der Burg MEL, Lacave AJ, Panici PB, Kenter GG, Casado A, Mendiola C, Coens C, Verleye L, Stuart GCE, Pecorelli S, Reed NS, Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer., N. Engl. J. Med 363 (2010) 943–953. doi:10.1056/NEJMoa0908806. - DOI - PubMed
-
- Kehoe S, Hook J, Nankivell M, Jayson GC, Kitchener H, Lopes T, Luesley D, Perren T, Bannoo S, Mascarenhas M, Dobbs S, Essapen S, Twigg J, Herod J, McCluggage G, Parmar M, Swart A-M, Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial, Lancet. 386 (2015) 249–257. doi:10.1016/S0140-6736(14)62223-6. - DOI - PubMed
-
- Onda T, Satoh T, Ogawa G, Saito T, Kasamatsu T, Nakanishi T, Mizutani T, Takehara K, Okamoto A, Ushijima K, Kobayashi H, Kawana K, Yokota H, Takano M, Kanao H, Watanabe Y, Yamamoto K, Yaegashi N, Kamura T, Yoshikawa H, Comparison of survival between primary debulking surgery and neoadjuvant chemotherapy for stage III/IV ovarian, tubal and peritoneal cancers in phase III randomised trial, Eur. J. Cancer 130 (2020) 114–125. doi:10.1016/j.ejca.2020.02.020. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials