Phase I Study of Muscadine Grape Extract for Patients With Advanced Cancer
- PMID: 33867481
- PMCID: PMC8141001
- DOI: 10.1097/COC.0000000000000814
Phase I Study of Muscadine Grape Extract for Patients With Advanced Cancer
Abstract
Objective: Preclinical studies with muscadine grape extract (MGE) show antitumor activity and decreased systemic inflammation. This phase I study (NCT02583269) assessed safety and tolerability of a proprietary MGE preparation in patients with advanced solid tumors.
Methods: Patients with metastatic or unresectable cancers who were progressing on standard therapies were assigned to MGE in a standard 3+3 design. Five dose levels were tested (320 to 1600 mg total phenolics/d). Safety and maximum-tolerated dose were assessed after 4 weeks. Patients were evaluated for response at 8 weeks and continued on MGE if clinically stable. Secondary outcomes were response, survival, adherence, fatigue, and quality of life (QOL).
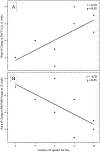
Results: In total, 23 patients (lung, n=7; gastrointestinal, n=7; genitourinary, n=6; other, n=3) received MGE capsules by mouth twice daily. The cohort [median age 72 years, 48% Eastern Cooperative Oncology Group (ECOG) 2] was heavily pretreated. After 4 weeks on MGE, possibly attributable adverse events grade 2 or higher were fatigue (n=1), decreased lymphocyte count (n=1), and constipation (n=2), including 1 dose-limiting toxicity for grade 3 constipation. Maximum-tolerated dose was not reached. No partial responses were observed. Median time on therapy was 8 weeks, with 29% of patients treated beyond 16 weeks and a median overall survival of 7.2 months. QOL and fatigue levels were stable from baseline to 8 weeks. Higher MGE dose was correlated with improvement in self-reported physical well-being QOL at 8 weeks (r=0.6; P=0.04).
Conclusions: MGE is safe and well-tolerated in heavily pretreated and older cancer patients. The potential anticancer properties and the effects of MGE on physical well-being and QOL metrics will be evaluated in future studies.
Copyright © 2021 Wolters Kluwer Health, Inc. All rights reserved.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Yang J, Xiao YY. Grape phytochemicals and associated health benefits. Critical reviews in food science and nutrition. 2013;53(11):1202–1225. - PubMed
-
- Lee JH, Johnson JV, Talcott ST. Identification of ellagic acid conjugates and other polyphenolics in muscadine grapes by HPLC-ESI-MS. Journal of agricultural and food chemistry. 2005;53(15):6003–6010. - PubMed
-
- Yi W, Fischer J, Akoh CC. Study of anticancer activities of muscadine grape phenolics in vitro. Journal of agricultural and food chemistry. 2005;53(22):8804–8812. - PubMed
-
- Mertens-Talcott SU, Lee JH, Percival SS, Talcott ST. Induction of cell death in Caco-2 human colon carcinoma cells by ellagic acid rich fractions from muscadine grapes (Vitis rotundifolia). Journal of agricultural and food chemistry. 2006;54(15):5336–5343. - PubMed
-
- God JM, Tate P, Larcom LL. Anticancer effects of four varieties of muscadine grape. Journal of medicinal food. 2007;10(1):54–59. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical