The role of Fibrinogen-like proteins in Cancer
- PMID: 33867830
- PMCID: PMC8040309
- DOI: 10.7150/ijbs.56748
The role of Fibrinogen-like proteins in Cancer
Abstract
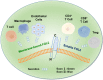
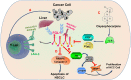
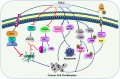
Fibrinogen-associated protein (FREP) family is a family of proteins with a fibrin domain at the carboxyl terminus. Recent investigations illustrated that two members of FREP family, fibrinogen-like protein-1 (FGL1) and fibrinogen-like protein-2 (FGL2), play crucial roles in cancer by regulating the proliferation, invasion, and migration of tumor cells, or regulating the functions of immune cells in tumor microenvironment. Meanwhile, they are potential targets for medical intervention of tumor development. In this review, we discussed the structure, and the roles of FGL1 and FGL2 in tumors, especially the roles in regulating immune cell functions.
Keywords: Cancer; FGF1; FGF2.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

References
-
- Yee VC, Pratt KP, Côté HC, Trong IL, Chung DW, Davie EW. et al. Crystal structure of a 30 kDa C-terminal fragment from the gamma chain of human fibrinogen. Structure (London, England: 1993) 1997;5:125–38. - PubMed
-
- Li D, Nie H, Jiang K, Li N, Huo Z, Yan X. Molecular characterization and expression analysis of fibrinogen related protein (FREP) genes of Manila clam (Ruditapes philippinarum) after lipopolysaccharides challenge. Comp Biochem Physiol C Toxicol Pharmacol. 2020;228:108672. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

