Construction of N-Boc-2-Alkylaminoquinazolin-4(3 H)-Ones via a Three-Component, One-Pot Protocol Mediated by Copper(II) Chloride that Spares Enantiomeric Purity
- PMID: 33867902
- PMCID: PMC8048503
- DOI: 10.1002/adsc.202001279
Construction of N-Boc-2-Alkylaminoquinazolin-4(3 H)-Ones via a Three-Component, One-Pot Protocol Mediated by Copper(II) Chloride that Spares Enantiomeric Purity
Abstract
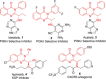
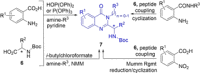
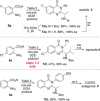
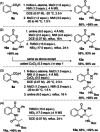
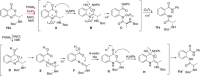
Chiral 2-alkylquinazolinones are key synthetic intermediates, but their preparation in high optical purity is challenging. Thus, a multicomponent procedure integrating anthranilic acids, N-Boc-amino acids, and amines in the presence of methanesulfonyl chloride, N-methylimidazole, and copper(II) chloride was developed to mildly afford N-Boc-2-alkylaminoquinazolin-4(3H)-ones with excellent preservation of enantiomeric purity (>99% ee). Copper(II) chloride was essential to retaining enantiopurity, and reaction component structural changes were well tolerated, resulting in an efficient, all-in-one procedure that promotes sequential coupling, lactonization, aminolysis, and cyclization in good yields. The method was applied to the rapid assembly of four key intermediates used in the synthesis of high profile quinazolinones, including several PI3K inhibitor drugs.
Keywords: PI3 kinase; copper-mediated; enantiopurity; quinazolinone; racemization.
© 2021 The Authors. Advanced Synthesis & Catalysis published by Wiley-VCH GmbH.
Figures

References
-
- None
-
- Resende D. I. S. P., Boonpothong P., Sousa E., Kijjoa A., Pinto M. M. M., Nat. Prod. Rep. 2019, 36, 7–34; - PubMed
-
- Khan I., Ibrar A., Ahmed W., Saeed A., Eur. J. Med. Chem. 2015, 90, 124–169; - PubMed
-
- Kshirsagar U. A., Org. Biomol. Chem. 2015, 13, 9336–9352; - PubMed
-
- Demeunynck M., Baussanne I., Curr. Med. Chem. 2013, 20, 794–814; - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
