Nitric Oxide in the Spinal Cord Is Involved in the Hyperalgesia Induced by Tetrahydrobiopterin in Chronic Restraint Stress Rats
- PMID: 33867911
- PMCID: PMC8044835
- DOI: 10.3389/fnins.2021.593654
Nitric Oxide in the Spinal Cord Is Involved in the Hyperalgesia Induced by Tetrahydrobiopterin in Chronic Restraint Stress Rats
Abstract
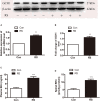
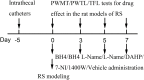
It has been well recognized that exposure to chronic stress could increase pain responding and exacerbate pain symptoms, resulting in stress-induced hyperalgesia. However, the mechanisms underlying stress-induced hyperalgesia are not yet fully elucidated. To this end, we observed that restraint as a stressful event exacerbated mechanical and thermal hyperalgesia, accompanied with up-regulation of nitric oxide (NO) (P < 0.001), GTP cyclohydrolase 1 (GCH1) (GCH1 mRNA: P = 0.001; GCH1 protein: P = 0.001), and tetrahydrobiopterin (BH4) concentration (plasma BH4: P < 0.001; spinal BH4: P < 0.001) on Day 7 in restraint stress (RS) rats. Intrathecal injection of N ω-nitro-L-arginine methyl ester (L-NAME), a non-specific NO synthase inhibitor, or N-([3-(aminomethyl)phenyl]methyl) ethanimidamide, a special inhibitor of inducible NO synthase (iNOS), for seven consecutive days attenuated stress-induced hyperalgesia and decreased the production of NO (P < 0.001). Interestingly, 7-nitro indazole, a special inhibitor of neuronal NO synthase, alleviated stress-induced hyperalgesia but did not affect spinal NO synthesis. Furthermore, intrathecal injection of BH4 not only aggravated stress-induced hyperalgesia but also up-regulated the expression of spinal iNOS (iNOS mRNA: P = 0.015; iNOS protein: P < 0.001) and NO production (P < 0.001). These findings suggest that hyperalgesia induced by RS is associated with the modulation of the GCH1-BH4 system and constitutively expressed spinal iNOS. Thus, the GCH1-BH4-iNOS signaling pathway may be a new novel therapeutic target for pain relief in the spinal cord.
Keywords: GTP cyclohydrolase 1; inducible nitric oxide synthase; spinal cord; stress-induced hyperalgesia; tetrahydrobiopterin.
Copyright © 2021 Huang, Jiao, Zhu, Xiong, Lu, Ai, Yang, Zhao and Xu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

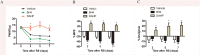




Similar articles
-
MiR-124 inhibits spinal neuronal apoptosis through binding to GCH1.Eur Rev Med Pharmacol Sci. 2019 Jun;23(11):4564-4574. doi: 10.26355/eurrev_201906_18032. Eur Rev Med Pharmacol Sci. 2019. PMID: 31210282
-
Inhibition of p38 mitogen-activated protein kinase attenuates interleukin-1beta-induced thermal hyperalgesia and inducible nitric oxide synthase expression in the spinal cord.J Neurochem. 2005 Aug;94(3):742-52. doi: 10.1111/j.1471-4159.2005.03226.x. J Neurochem. 2005. PMID: 16033422
-
Intact carrageenan-induced thermal hyperalgesia in mice lacking inducible nitric oxide synthase.Neuroscience. 2003;120(3):847-54. doi: 10.1016/s0306-4522(03)00362-2. Neuroscience. 2003. PMID: 12895524
-
GCH1 variants, tetrahydrobiopterin and their effects on pain sensitivity.Scand J Pain. 2014 Apr 1;5(2):121-128. doi: 10.1016/j.sjpain.2013.12.001. Scand J Pain. 2014. PMID: 29913682 Review.
-
GTP cyclohydroxylase1 (GCH1): Role in neurodegenerative diseases.Gene. 2023 Dec 20;888:147749. doi: 10.1016/j.gene.2023.147749. Epub 2023 Aug 29. Gene. 2023. PMID: 37652170 Review.
Cited by
-
Interactions among Endothelial Nitric Oxide Synthase, Cardiovascular System, and Nociception during Physiological and Pathophysiological States.Molecules. 2022 Apr 29;27(9):2835. doi: 10.3390/molecules27092835. Molecules. 2022. PMID: 35566185 Free PMC article. Review.
-
Targeting the nitric oxide/cGMP signaling pathway to treat chronic pain.Neural Regen Res. 2023 May;18(5):996-1003. doi: 10.4103/1673-5374.355748. Neural Regen Res. 2023. PMID: 36254980 Free PMC article. Review.
-
Resolution of Lipopolysaccharide-Induced Inflammation Followed by DNA Hypomethylation and Increased Tetrahydrobiopterin Biosynthesis in Mouse Hippocampus.Brain Sci. 2025 Aug 18;15(8):880. doi: 10.3390/brainsci15080880. Brain Sci. 2025. PMID: 40867210 Free PMC article.
-
Multifaceted role of nitric oxide in vascular dementia.Med Gas Res. 2025 Dec 1;15(4):496-506. doi: 10.4103/mgr.MEDGASRES-D-24-00158. Epub 2025 Apr 29. Med Gas Res. 2025. PMID: 40300885 Free PMC article. Review.
References
-
- Deciga-Campos M., Navarrete-Vazquez G., Lopez-Munoz F. J., Librowski T., Sanchez-Recillas A., Yanez-Perez V., et al. (2016). Pharmacological profile of N-(2,6-dichlorophenyl)-2-(4-methyl-1-piperidinyl)acetamide, a novel analogue of lidocaine. Life Sci. 155 48–55. 10.1016/j.lfs.2016.05.015 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

