Peripheral Electrical Stimulation Modulates Cortical Beta-Band Activity
- PMID: 33867919
- PMCID: PMC8044771
- DOI: 10.3389/fnins.2021.632234
Peripheral Electrical Stimulation Modulates Cortical Beta-Band Activity
Abstract
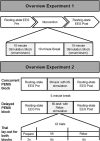
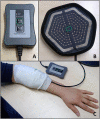
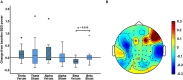
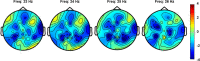
Low-frequency peripheral electrical stimulation using a matrix electrode (PEMS) modulates spinal nociceptive pathways. However, the effects of this intervention on cortical oscillatory activity have not been assessed yet. The aim of this study was to investigate the effects of low-frequency PEMS (4 Hz) on cortical oscillatory activity in different brain states in healthy pain-free participants. In experiment 1, PEMS was compared to sham stimulation. In experiment 2, motor imagery (MI) was used to modulate the sensorimotor brain state. PEMS was applied either during MI-induced oscillatory desynchronization (concurrent PEMS) or after MI (delayed PEMS) in a cross-over design. For both experiments, PEMS was applied on the left forearm and resting-state electroencephalography (EEG) was recording before and after each stimulation condition. Experiment 1 showed a significant decrease of global resting-state beta power after PEMS compared to sham (p = 0.016), with a median change from baseline of -16% for PEMS and -0.54% for sham. A cluster-based permutation test showed a significant difference in resting-state beta power comparing pre- and post-PEMS (p = 0.018) that was most pronounced over bilateral central and left frontal sensors. Experiment 2 did not identify a significant difference in the change from baseline of global EEG power for concurrent PEMS compared to delayed PEMS. Two cluster-based permutation tests suggested that frontal beta power may be increased following both concurrent and delayed PEMS. This study provides novel evidence for supraspinal effects of low-frequency PEMS and an initial indication that the presence of a cognitive task such as MI may influence the effects of PEMS on beta activity. Chronic pain has been associated with changes in beta activity, in particular an increase of beta power in frontal regions. Thus, brain state-dependent PEMS may offer a novel approach to the treatment of chronic pain. However, further studies are warranted to investigate optimal stimulation conditions to achieve a reduction of pain.
Keywords: electroencephalography; nociception; peripheral electrical stimulation; sensorimotor rhythm; state-dependent stimulation.
Copyright © 2021 Arendsen, Guggenberger, Zimmer, Weigl and Gharabaghi.
Conflict of interest statement
TW is the founder and CEO of Bomedus GmbH, the company that developed the stimulation device used in this study. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer EL-L declared a past co-authorship with one of the authors AG to the handling editor.
Figures
Similar articles
-
Recruitment of Additional Corticospinal Pathways in the Human Brain with State-Dependent Paired Associative Stimulation.J Neurosci. 2018 Feb 7;38(6):1396-1407. doi: 10.1523/JNEUROSCI.2893-17.2017. Epub 2018 Jan 15. J Neurosci. 2018. PMID: 29335359 Free PMC article.
-
Electrical stimulation of the frontal cortex enhances slow-frequency EEG activity and sleepiness.Neuroscience. 2016 Jun 2;324:119-30. doi: 10.1016/j.neuroscience.2016.03.007. Epub 2016 Mar 8. Neuroscience. 2016. PMID: 26964682
-
Relieving peripheral neuropathic pain by increasing the power-ratio of low-β over high-β activities in the central cortical region with EEG-based neurofeedback: Study protocol for a controlled pilot trial (SMRPain study).Neurophysiol Clin. 2020 Feb;50(1):5-20. doi: 10.1016/j.neucli.2019.12.002. Epub 2020 Feb 8. Neurophysiol Clin. 2020. PMID: 32046899
-
ERD/ERS patterns reflecting sensorimotor activation and deactivation.Prog Brain Res. 2006;159:211-22. doi: 10.1016/S0079-6123(06)59014-4. Prog Brain Res. 2006. PMID: 17071233 Review.
-
Sensorimotor and cognitive involvement of the beta-gamma oscillation in the frontal N30 component of somatosensory evoked potentials.Neuropsychologia. 2015 Dec;79(Pt B):215-22. doi: 10.1016/j.neuropsychologia.2015.04.033. Epub 2015 May 19. Neuropsychologia. 2015. PMID: 26002756 Review.
Cited by
-
Repetitive Peripheral Magnetic Stimulation Combined with Motor Imagery Changes Resting-State EEG Activity: A Randomized Controlled Trial.Brain Sci. 2022 Nov 15;12(11):1548. doi: 10.3390/brainsci12111548. Brain Sci. 2022. PMID: 36421872 Free PMC article.
-
Towards the Objective Identification of the Presence of Pain Based on Electroencephalography Signals' Analysis: A Proof-of-Concept.Sensors (Basel). 2022 Aug 20;22(16):6272. doi: 10.3390/s22166272. Sensors (Basel). 2022. PMID: 36016032 Free PMC article.
-
Whole-Body Adaptive Functional Electrical Stimulation Kinesitherapy Can Promote the Restoring of Physiological Muscle Synergies for Neurological Patients.Sensors (Basel). 2022 Feb 13;22(4):1443. doi: 10.3390/s22041443. Sensors (Basel). 2022. PMID: 35214345 Free PMC article.
-
Effect of Implantable Electrical Nerve Stimulation on Cortical Dynamics in Patients With Herpes Zoster-Related Pain: A Prospective Pilot Study.Front Bioeng Biotechnol. 2022 May 16;10:862353. doi: 10.3389/fbioe.2022.862353. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35651542 Free PMC article.
-
The Potential of Electrical Stimulation and Smart Textiles for Patients with Diabetes Mellitus.Horm Metab Res. 2022 Sep;54(9):583-586. doi: 10.1055/a-1892-6489. Epub 2022 Jul 6. Horm Metab Res. 2022. PMID: 35793708 Free PMC article.
References
-
- Arendsen L. J., Hugh-Jones S., Lloyd D. M. (2018). Transcranial alternating current stimulation at alpha frequency reduces pain when the intensity of pain is uncertain. J. Pain 19 807–818. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

