Sustained Hippocampal Synaptic Pathophysiology Following Single and Repeated Closed-Head Concussive Impacts
- PMID: 33867941
- PMCID: PMC8044326
- DOI: 10.3389/fncel.2021.652721
Sustained Hippocampal Synaptic Pathophysiology Following Single and Repeated Closed-Head Concussive Impacts
Abstract
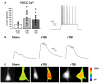
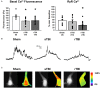
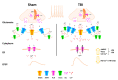
Traumatic brain injury (TBI), and related diseases such as chronic traumatic encephalopathy (CTE) and Alzheimer's (AD), are of increasing concern in part due to enhanced awareness of their long-term neurological effects on memory and behavior. Repeated concussions, vs. single concussions, have been shown to result in worsened and sustained symptoms including impaired cognition and histopathology. To assess and compare the persistent effects of single or repeated concussive impacts on mediators of memory encoding such as synaptic transmission, plasticity, and cellular Ca2+ signaling, a closed-head controlled cortical impact (CCI) approach was used which closely replicates the mode of injury in clinical cases. Adult male rats received a sham procedure, a single impact, or three successive impacts at 48-hour intervals. After 30 days, hippocampal slices were prepared for electrophysiological recordings and 2-photon Ca2+ imaging, or fixed and immunostained for pathogenic phospho-tau species. In both concussion groups, hippocampal circuits showed hyper-excitable synaptic responsivity upon Schaffer collateral stimulation compared to sham animals, indicating sustained defects in hippocampal circuitry. This was not accompanied by sustained LTP deficits, but resting Ca2+ levels and voltage-gated Ca2+ signals were elevated in both concussion groups, while ryanodine receptor-evoked Ca2+ responses decreased with repeat concussions. Furthermore, pathogenic phospho-tau staining was progressively elevated in both concussion groups, with spreading beyond the hemisphere of injury, consistent with CTE. Thus, single and repeated concussions lead to a persistent upregulation of excitatory hippocampal synapses, possibly through changes in postsynaptic Ca2+ signaling/regulation, which may contribute to histopathology and detrimental long-term cognitive symptoms.
Keywords: CTE; Ca2+; LTP; concussion; hippocampus; synaptic transmission; tau.
Copyright © 2021 McDaid, Briggs, Barrington, Peterson, Kozlowski and Stutzmann.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Traumatic brain injury (TBI) in collision sports: Possible mechanisms of transformation into chronic traumatic encephalopathy (CTE).Metabolism. 2019 Nov;100S:153943. doi: 10.1016/j.metabol.2019.07.007. Metabolism. 2019. PMID: 31610856 Review.
-
Reduced presynaptic vesicle stores mediate cellular and network plasticity defects in an early-stage mouse model of Alzheimer's disease.Mol Neurodegener. 2019 Jan 22;14(1):7. doi: 10.1186/s13024-019-0307-7. Mol Neurodegener. 2019. PMID: 30670054 Free PMC article.
-
Chronic Cognitive Deficits and Associated Histopathology Following Closed-Head Concussive Injury in Rats.Front Neurol. 2019 Jul 2;10:699. doi: 10.3389/fneur.2019.00699. eCollection 2019. Front Neurol. 2019. PMID: 31312174 Free PMC article.
-
American Medical Society for Sports Medicine position statement: concussion in sport.Br J Sports Med. 2013 Jan;47(1):15-26. doi: 10.1136/bjsports-2012-091941. Br J Sports Med. 2013. PMID: 23243113 Review.
-
Repetitive mild traumatic brain injury induces persistent alterations in spontaneous synaptic activity of hippocampal CA1 pyramidal neurons.IBRO Neurosci Rep. 2022 Feb 9;12:157-162. doi: 10.1016/j.ibneur.2022.02.002. eCollection 2022 Jun. IBRO Neurosci Rep. 2022. PMID: 35746968 Free PMC article.
Cited by
-
Single Neuron Modeling Identifies Potassium Channel Modulation as Potential Target for Repetitive Head Impacts.Neuroinformatics. 2023 Jul;21(3):501-516. doi: 10.1007/s12021-023-09633-7. Epub 2023 Jun 9. Neuroinformatics. 2023. PMID: 37294503 Free PMC article.
-
Repetitive concussions promote microglia-mediated engulfment of presynaptic excitatory input associated with cognitive dysfunction.Commun Biol. 2025 Feb 28;8(1):335. doi: 10.1038/s42003-025-07729-1. Commun Biol. 2025. PMID: 40021832 Free PMC article.
-
Fecal microbiota transplantation inhibited neuroinflammation of traumatic brain injury in mice via regulating the gut-brain axis.Front Cell Infect Microbiol. 2023 Sep 7;13:1254610. doi: 10.3389/fcimb.2023.1254610. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37743861 Free PMC article.
-
Maternal choline supplementation protects against age-associated cholinergic and GABAergic basal forebrain neuron degeneration in the Ts65Dn mouse model of Down syndrome and Alzheimer's disease.Neurobiol Dis. 2023 Nov;188:106332. doi: 10.1016/j.nbd.2023.106332. Epub 2023 Oct 26. Neurobiol Dis. 2023. PMID: 37890559 Free PMC article.
-
Brainstem and Cortical Spreading Depolarization in a Closed Head Injury Rat Model.Int J Mol Sci. 2021 Oct 28;22(21):11642. doi: 10.3390/ijms222111642. Int J Mol Sci. 2021. PMID: 34769073 Free PMC article.
References
-
- Ahmed S. M., Weber J. T., Liang S., Willoughby K. A., Sitterding H. A., Rzigalinski B. A., et al. . (2002). NMDA receptor activation contributes to a portion of the decreased mitochondrial membrane potential and elevated intracellular free calcium in strain-injured neurons. J. Neurotrauma 19, 1619–1629. 10.1089/089771502762300274 - DOI - PubMed
-
- Almeida-Suhett C. P., Prager E. M., Pidoplichko V., Figueiredo T. H., Marini A. M., Li Z., et al. . (2015). GABAergic interneuronal loss and reduced inhibitory synaptic transmission in the hippocampal CA1 region after mild traumatic brain injury. Exp. Neurol. 273, 11–23. 10.1016/j.expneurol.2015.07.028 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

