Transduction and Adaptation Mechanisms in the Cilium or Microvilli of Photoreceptors and Olfactory Receptors From Insects to Humans
- PMID: 33867944
- PMCID: PMC8046925
- DOI: 10.3389/fncel.2021.662453
Transduction and Adaptation Mechanisms in the Cilium or Microvilli of Photoreceptors and Olfactory Receptors From Insects to Humans
Abstract
Sensing changes in the environment is crucial for survival. Animals from invertebrates to vertebrates use both visual and olfactory stimuli to direct survival behaviors including identification of food sources, finding mates, and predator avoidance. In primary sensory neurons there are signal transduction mechanisms that convert chemical or light signals into an electrical response through ligand binding or photoactivation of a receptor, that can be propagated to the olfactory and visual centers of the brain to create a perception of the odor and visual landscapes surrounding us. The fundamental principles of olfactory and phototransduction pathways within vertebrates are somewhat analogous. Signal transduction in both systems takes place in the ciliary sub-compartments of the sensory cells and relies upon the activation of G protein-coupled receptors (GPCRs) to close cyclic nucleotide-gated (CNG) cation channels in photoreceptors to produce a hyperpolarization of the cell, or in olfactory sensory neurons open CNG channels to produce a depolarization. However, while invertebrate phototransduction also involves GPCRs, invertebrate photoreceptors can be either ciliary and/or microvillar with hyperpolarizing and depolarizing responses to light, respectively. Moreover, olfactory transduction in invertebrates may be a mixture of metabotropic G protein and ionotropic signaling pathways. This review will highlight differences of the visual and olfactory transduction mechanisms between vertebrates and invertebrates, focusing on the implications to the gain of the transduction processes, and how they are modulated to allow detection of small changes in odor concentration and light intensity over a wide range of background stimulus levels.
Keywords: activation; adaptation; inactivation; olfaction; phototransduction cascade.
Copyright © 2021 Abbas and Vinberg.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
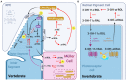
Figures





References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

